
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Can I get help solving these reactions? Thank you!
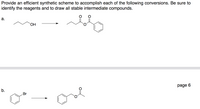
Transcribed Image Text:Provide an efficient synthetic scheme to accomplish each of the following conversions. Be sure to
identify the reagents and to draw all stable intermediate compounds.
а.
HO.
page 6
b.
Br
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- From the list of reactions below, pick out the combustion reactions? 1) 2C2H6(g) + 7O2 (g) → 4CO2(g) + 6H2Ol) 2) MgO(s) + CO2 (g) → MgCO3(s) 3) ZnCO3(s) → ZnO (s) + CO2 (g) 4) 2CH3OH(l) + 3O2 (g) → 2CO2(g) + 4H2O(l) a 1, 2, 3, and 4 b 3 and 4 c 2, 3, and 4 d 1, 3, and 4 e 1 and 4arrow_forwardWhen answering this problem, report the answer with the appropriate number of significant figures. When entering units, use proper abbreviated units with proper capitalization. A student followed the procedure in this experiment, using 48.92 mL of 1.00 molar HCl and 51.08 mL of 1.00 molar NaOH. Analysis of the collected data resulted in a heat transfer of 2808 J during reaction. Use this information to determine the heat of neutralization for this reaction in kJ/mol. Note: Your answer is assumed to be reduced to the highest power possible. Your Answer: ___________*10___ Units______arrow_forwardPlease helparrow_forward
- Here is some information to help. In this experiment, magnesium metal is heated until it reacts with oxygen in the air according to the following balanced equation: 2Mg(s)+ O2(g) → 2MgO(s) (silver metal) (white-gray ash) As the magnesium burns in air, it may also combine with nitrogen in air. To remove any nitride product, water is added, and the product is reheated. Any nitride product is converted to magnesium oxide and ammonia. 3Mg(s)+ N2(g) → Mg3N2(s) Mg3N2(s)+3H2O(l)→3MgO(s)+2NH3(aq) A weighed amount of the magnesium metal is used, and the mass of the oxide product formed is determined at the end of the experiment. The mass of oxygen that combines with the magnesium is obtained from the difference between the mass of the oxide product and the original mass of magnesium. mass of oxide product-mass of magnesium=mass of oxygen in oxide product The empirical formula of the oxide…arrow_forwardPLEASE HELParrow_forwardPredict the products of the reaction below. That is, complete the right-hand side of the chemical equation. Be sure your equation is balanced. HI + NaOH → I Don't Know 1841 Submit 3 E 5 T 6 G 0-0 X S stv♫ 5 © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Cer A all 9 zoomarrow_forward
- I need help understanding how to do this homework question. I've been out sick so I don't even know how to even begin to attempt this question.arrow_forwardPlease help me solve thisarrow_forwardIdentify the type of reaction that each material must undergo to generate water. LiOH₍s₎ H₂SO₄₍aq₎ H₂₍g₎ + O₂₍g₎ CH₄₍g₎ Which material would you recommend for the kit?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY