
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
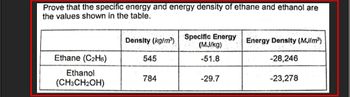
Transcribed Image Text:Prove that the specific energy and energy density of ethane and ethanol are
the values shown in the table.
Ethane (C₂H6)
Ethanol
(CH3CH₂OH)
Density (kg/m³) Specific Energy
(MJ/kg)
-51.8
545
784
-29.7
Energy Density (MJ/m³)
-28,246
-23,278
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The complete combustion of acetylene, C2H2(g), produces 1300. kJ of energy per mole of acetylene consumed. How many grams of acetylene must be burned to produce enough heat to raise the temperature of 1.05 gal water by 10.8°C if the process is 85.6% efficient? Assume the density of water is 1.00g/cubic meterarrow_forwardThe combustion of 0.1564 g benzoic acid increases the temperature of a bomb calorimeter by 2.53°C. Calculate the heat capacity of this calorimeter. (The energy released by the combustion of benzoic acid is 26.42 kJ/g.) Heat capacity = __________ kJ/°C A 0.2137-g sample of vanillin (C8H8O3) is then burned in the same calorimeter, and the temperature increases by 3.22°C. What is the energy of combustion per gram of vanillin? Energy =__________ kJ/g Per mole of vanillin? Energy = ___________ kJ/molarrow_forwardUse the following information to answer Questions 6-9: A sample of NH4NO3 with a mass of 5.0 g is dissolved in 40.1 g of deoinized water in a calorimeter. The initial temperature of the water is 21.2 oC. The final temperature of the solution is 15.4 oC. The calorimeter constant = 25.2 J/oC. If qH2O = -910 J and qcal = -130 J for 5.0 g of NH4NO3, calculate the heat of solution in J per gram of NH4NO3.arrow_forward
- Given the following thermochemical equation: 4NH3(g) + 502(g) → 4NO(g) + 6H2O(g) Calculate the enthalpy change when 2.50 g NH3(g) is reacted with excess oxygen. AH = -906 kJarrow_forwardYou wish to find the enthalpy for the reaction 6 Gel (s) + 14 NH:1 (s) → 3 Ge:H6 (1) + 7 N2 (g) + 38 HI (g) kJ/mol Given the following equations Equation 1: 2 Ge (s) + 3 H2 (g) → Ge:H6 (1) AH = 137.3 kJ/mol Equation 2: Ge (s) + 4 HI (g) – Gel: (s) + 2 H2(g) AH = -247.8 kJ/mol Equation 3: 2 NH&| (s) → N2 (g) + 2 HI (g) + 3 H2(g) AH = 455.8 kJ/mol 2 4 C What would be the enthalpy change, in kJ/mol, for 6 Gel4 (s) + 12 H2(g) - 6 Ge (s) + 24 HI (g)? 7 8 9. +/- x 100 3. 1,arrow_forwardIf a 50.0g sample of Cu is heated to 180.00C and is placed in 100.0 ml of water in a calorimeter at 25.00C, what will the final temperature be for the system? Assume that all of the heat goes from the copper to the water. (None is lost to the surroundings or calorimeter.) dH20 =1.00g/ml C Cu = 0.385J/goC CH2O = 4.184 J/goCarrow_forward
- Using bond energies determine the heat generated when 32 grams of methane, CH4, is burned. (in kJ) CH4 + O2 → CO2 + H2Oarrow_forwardExplain it and answer it correctly.arrow_forwardA scientist measures the standard enthalpy change for this reaction to be 2863.2 kJ/mol. 6CO2(g) + 6 H2O(1) C6H1206 + 6 O2(g) Based on this value and the standard formation enthalpies for the other substances, the standard formation enthalpy of H20(1) is kJ/mol.arrow_forward
- Using bond energies determine the heat generated when 32 grams of methane, CH4, is burned. (in kJ) CH4 + O2 → CO2 + H2Oarrow_forwardcalculate calorimeter constant to 2 significant figuresarrow_forwardA chemist carefully measures the amount of heat needed to raise the temperature of a 533.0 mg sample of CH,N from 53.8 °C to 68.3 °C. The experiment shows that 16.5 J of heat are needed. What can the chemist report for the molar heat capacity of CH,N? Round your answer to 3 significant digits. -1 - 1 J•mol • K x10arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY