
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
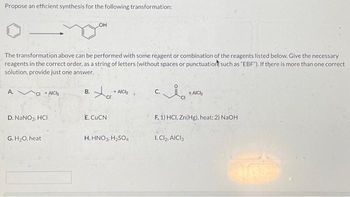
Transcribed Image Text:Propose an efficient synthesis for the following transformation:
The transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary
reagents in the correct order, as a string of letters (without spaces or punctuation such as "EBF"). If there is more than one correct
solution, provide just one answer.
A.
CI+AICH
D. NaNO₂, HCI
G. H₂O, heat
OH
B.
Xa
cr
E. CUCN
- AICH
H. HNO3, H₂SO4
C.
AICH
F. 1) HCI, Zn(Hg), heat; 2) NaOH
1. Cl₂, AICI 3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 7. Propose syntheses for the following compounds, starting with acetylene and any other reagents you might need. OH H. Brarrow_forwardPropose an efficient synthesis for the given reaction.arrow_forwardX Incorrect. Propose an efficient synthesis for the following transformation: NH₂ The transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary reagents in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct solution, provide just one answer. A. Fe, H3O+ D. NaNO₂, HCI G. NaOH, H₂O, heat CN $ CDH B. la CI E. CuBr H. CuCN + AICI3 C. ia. F. NaNH2 + pyridine I. Br2, FeBr3arrow_forward
- Propose an efficient synthesis for the following transformation: OMe S. S H The transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary reagents in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct solution, provide just one answer. SH A. OH + acid B. + acid C. HS HO j + + acid D. H3O+, heat E. [H+], CH3NH2 F. 1) Et2CuLi; 2) H3O+ G. Na2Cr2O7, H2SO4, H2O H. 1) LIAI(OR)3H; 2) H3O+ I. 1) LiAlH4; 2) H3O+ J. SOCI 2 K. PCC or DMP L. RCO3Harrow_forwardPropose an efficient synthesis for the following transformation: (g) Eto The transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary reagents in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct solution, provide just one answer. A. H3O+ D. PCC or DMP OEt B. cat. acid (-H₂O) E. xs LiAlH4 С. НО F. HO OH OH + cat. H₂SO4 + NaHarrow_forwardFrom the table of available reagents select the one(s) you would use to accomplish the transformations shown below. Use the minimum number of steps; in no case are more than two steps necessary. List reagents by letter in the order that they are used.arrow_forward
- Identify reagents that can be used to achieve the following transformation): OH он The transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary reagents in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct solution, provide just one answer. A. LDA, -78°C B. NazCr207, H2SO4, H2O C. PCC or DMP D. NaH, 25°C E. 1) EtMgBr; 2) H3O* F. CH3CH2B. G. SOCI2 Н. 1) LIAIH4: 2) HзО* I. CH3Iarrow_forwardPropose an efficient synthesis for the following transformation: -CN The transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary reagent(s) in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct solution, provide just one answer. A B HBr, ROOR D HBr G Br2, hv 1) xs NaNH, 2) H₂O E 1) BH 3 THF 2) H₂O2, NaOH H NaOEt C TsCl, pyridine F t-BuOK I NaCNarrow_forwardPropose an efficient synthesis for the following transformation: OH H. The transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary reagent(s) in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct solution, provide just one answer. A NaOH 1) MeMgBr; 2) H3O* Na2Cr2O7, H2SO4, H20 t-BUOK F G H. H2, Lindlar's cat. 1) O3; 2) DMS TSCI, py dilute H2SO4 K 1) LIAIH4; 2) DMP or PCC 1) EtMgBr; 2) H3O* 1) BH3-THF; 2) H2O2, NaOH H3O*arrow_forward
- Propose an efficient synthesis for the following transformation: The transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary reagents in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct solution, provide just one answer. A. -COOH HO OH + acid D. H3O+, heat G. Na2Cr2O7, H₂SO4, H₂O J. SOCI₂ B. HS SH + acid E. [H*], CH3NH2 H. 1) LIAI(OR) 3H; 2) H3O+ K. PCC or DMP 요 C. + acid F. 1) Et₂CuLi; 2) H3O+ I.1) LIAIH4; 2) H3O+ L. RCO 3Harrow_forwardPropose an efficient synthesis for the following transformation: OH OH The transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary reagents in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct solution, provide just one answer. A. SOCI₂ D. PCC or DMP B. 1) CH3CH₂MgBr; 2) H3O+ E. (CH3CH₂)2CuLi C. 1) LIAI(OR) 3H; 2) H3O+ F. 1) LIAIH4; 2) H3O+arrow_forwardPropose an efficient synthesis for the following transformation: OH The transformations above can be performed with some combination of the reagents listed below. Give the necessary reagents in the correct order for each transformation, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct solution, provide just one answer. A. t-BuOK D. Br2 G. TsCl, pyridine B. NAOMe E. H₂, Lindlar's cat. H. 1) xs NaNH2; 2) H₂O C. conc. H₂SO4, heat F. H₂SO4, H₂O, HgSO4 I. 1) R₂BH; 2) H₂O2, NaOHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY