
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Give clear handwritten answer with explanation needed..give answer both sub parts if you not then don't give answer..I will give you upvote for both answer
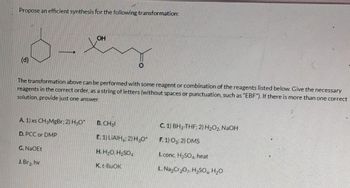
Transcribed Image Text:Propose an efficient synthesis for the following transformation:
(d)
A. 1) xs CH3MgBr; 2) H3O+
D. PCC or DMP
The transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary
reagents in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct
solution, provide just one answer.
G. NaOEt
OH
J. Br₂, hv
O
B. CH31
E. 1) LIAIH; 2) H₂O+
H. H₂O, H₂SO4
K. t-BuOK
C. 1) BH3-THF; 2) H₂O2, NaOH
F. 1) 03; 2) DMS
I. conc. H₂SO4, heat
L. Na₂Cr₂O7, H₂SO4 H₂O

Transcribed Image Text:Propose an efficient synthesis for the following transformation:
i - i
=
The transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary
reagents in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct
solution, provide just one answer.
A. CH3CH₂MgBr
D. NaNH,
B. CH3CH₂Br
E.H₂O+
C. HO
F.
HO
OH
+ cat. H₂SO4
OH + cat. NaH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- need help ASAP pls!arrow_forwardProblem (a) Answer the following questions regarding the F term for some electron configuration and its F2 level. (i) What is the S value for the term? (ii) What is the L value for the term? (iii) How many states are in the term? (iv) What is the multiplicity of the term? (v) What is the J value for the level? (vi) How many states are in the level? (b) What is the Clebsch-Gordon series for J based on L = 6 and S = 3/2?arrow_forwardAa.53.arrow_forward
- Solve for the entire table 2 show all work typed. Double-check your answers.arrow_forwardMolecule Ground state electron configuration NF (015) (01s*) NF+ (015) (01s*) (02) NF (015) (01s*) (025)" (025*) Answer Bank 1 2 3 4arrow_forwardWhy is 7.28 R and 7.29 S? Because both of the 4 is on a line, and I thought that the configuration would only switch when the lowest priority was on the wedge.arrow_forward
- Predict the shift for the protons attached to the atom indicated by the arrow. List the answer in ppm to the tenths place.arrow_forwardQUESTION #1 PROPOSE ANSWER FOR THE FOLLOWING SPECTRA ? AND JUSTIFY YOUR ANSWER 1- % abundance 100 60 50 40 30 20 10 مسلسل m/z ratio 1558arrow_forward(True or False): You can determine the identities of substances in a mixture by examining the peaks in an IR spectrum of the mixture. True Falsearrow_forward
- Please don't provide handwriting solutionarrow_forwardAnalyze the Proton NMR. Determine the number of signals and come up with possible chemical structures.arrow_forwardPart B What energetic degrees of freedom were considered for the atomic constituents? Check all that apply. rotational electronic translational vibrational Submit Request Answerarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY