
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
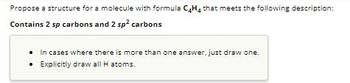
Transcribed Image Text:Propose a structure for a molecule with formula C4H4 that meets the following description:
Contains 2 sp carbons and 2 sp² carbons
• In cases where there is more than one answer, just draw one.
• Explicitly draw all H atoms.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Azides of heavy metals explode when struck sharply and are used in detonation caps. Draw the Lewis structure for the most stable azide ion, N3. Include lone pairs. Select / |||||| Draw N Templates Morearrow_forwardDraw the structural formula for the molecule that fits the following description. A small molecule with formula C₂H5O that contains an oxygen bonded to only one carbon. • Include all valence lone pairs in your answer. •You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one.arrow_forwardThe azide ion, N3, is a symmetrical ion, all of whose contributing structures have formal charges. Draw three important contributing Lewis structures for this ion. Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all nonbonding electrons. Show the formal charges of all atoms in the correct structure. Q Q Q ? ⒸNNNITY [] + F g i с H N S P Br CI | X Press [TAB] to move to the next option. Press (ALT+A) to get to the bonds and Morearrow_forward
- Draw Lewis structures and assign formal charges for the following compounds and ions. Note any resonance forms and explain which resonance form is the most important. Make a note of any other unusual features (such as "this one doesn't work" and why- what are the shortcomings of the model.) CO3²- CO NO₂ CNO (include isomers) Al₂Cl6 B₂H6 O2 (paramagnetic) H3O+ LiF IF7arrow_forwardConsider the structures below. Calculate the formal charge of each atom in each structure, then indicate which structure is the most accurate. Explain your choice and do NOT base it on the choice of central atom.arrow_forwardWhat is the formal charge on the carbon atom in methane, CH4? Report your answer as a whole number without any decimal places. Provide your answer below:arrow_forward
- Compute the formal charge (FC) on each atom in the following structures. (b) The hydronium ion, H3O+arrow_forwardPlease help answer thisarrow_forwardFill in the systematic names of the following chemical compounds. Note: for compounds containing hydrogen, you may give the common name instead. name of compound molecular formula | C1,0, CL,0, PCL, PCI, NH3arrow_forward
- Carbon monoxide (CO) has a very small dipole moment (0.122) despite having a polar C-O bond. Using resonance structures, please explain this conclusion.arrow_forwardPlease help mearrow_forwardDetermine the number of valence electrons in (CH₃C(O)CN) and then draw the corresponding Lewis structure.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY