
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
Propose a pathway for the following compound to enter gluconeogenesis / glycolysis. In your pathway, include enzymes (you can use E1, E2, etc.) as well as any necessary cofactors.
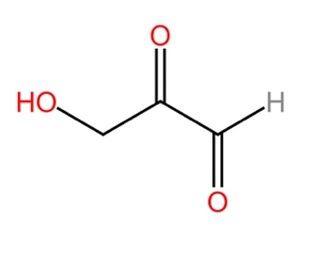
Transcribed Image Text:НО,
О
.H
I
Expert Solution
arrow_forward
Given compound
The compound given is not a common biomolecule or metabolite. It is methanolglyoxal , the alcoholic form of methylglyoxal. Methylglyoxal is a common metabolite found in the energy metabolism pathway of microbes. It can be converted to common intermediates like Pyruvate , DHAP (Dihydroxyacetone phosphate), etc.
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Draw OUT THE arrow pushing reaction mechanism for the following steps of glycolysis: SHOW/IDENTIFY the arrow pushing mechanism in at least 2 steps. CH,OPO CH,OH hexokinase 2+ H H H H H H ОН + ATP OH H ADP + H+ H ОН + НО HO OH H ОН H ОН Glucose-6-phosphate (G6P) Glucosearrow_forwardHow do the ligand (substrate) and the protein/enzyme predominantly interact? sigma bonds O Metallic bonds O Pi bonds O lonic bonds O Weak non-covalent bondsarrow_forwardConsider an alternative glycolysis pathway that starts with the phosphorylation of glucose to give glucose-6-phosphate. This (hypothetical) pathway exists in a (hypothetical) organism that does not express glucose-6-phosphate isomerase. Instead, the next step of this hypothetical pathway is a Glucose-6-Phosphate Aldolase. Draw the product or products that would be obtain by the reaction of Glucose – 6 – Phosphate with Glucose – 6 Phosphate Aldolase. Assume the reaction is completely irreversible. Explain in 1-3 sentences how you obtained your answerarrow_forward
- Prostaglandins are a class of eicosanoids, fatty acid derivatives with a variety of extremely potent actions on vertebrate issues. They are responsible for producing fever and inflammation and its associated pain. Prostaglandins are derived from he 20-carbon fatty acid arachidonic acid in a reaction catalyzed by the enzyme prostaglandin endoperoxide synthase. This enzyme, a cyclooxygenase, uses oxygen to convert arachidonic acid to PGG2, the immediate precursor of many different prostaglandins. Arachidonic Rate of formation Rate of formation of PGG2 with 10 mg/ml acid (mM) of PGG2 (mM/min) ibuprofen (mM/min) 0.285 21.3 13.3 0.342 24.0 15.4 0.513 30.3 20.6 0.855 38.4 28.2 2.00 49.8 41.5 The kinetic data given in the table are for the reaction catalyzed by a mutant of prostaglandin endoperoxide synthase. Focusing here on the first two columns, determine the Vmax and Km of the enzyme. mM/min Vmax mM Km buprofen is an inhibitor of prostaglandin endoperoxide synthase. By inhibiting the…arrow_forwardGlucose 6-phosphate is also a precursor for the synthesis of nucleotides that are used to build DNA and RNA. The first step of this pathway is catalyzed by an enzyme called glucose 6-phosphate dehydrogenase. Assume that reactions in this pathway with double arrows are reversible under cellular conditions, while those with single arrows are effectively irreversible. Which of the following enzymes, if present in your fructose-6-phosphate solution, would most substantially decrease the amount of 6-phosphogluconolactone you produce? Phosphoglucomutase G6P Dehydrogenase G6P Isomerase Hexokinase PFK-1arrow_forwardStep 4 of the pentose phosphate pathway converts ribulose-5-phosphate to ribose-5-phosphate. Which glycolytic reaction does this reaction resemble and what type of enzyme catalyzes it?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON