
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
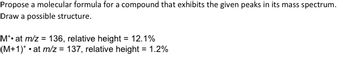
Transcribed Image Text:Propose a molecular formula for a compound that exhibits the given peaks in its mass spectrum.
Draw a possible structure.
M*• at m/z = 136, relative height = 12.1%
(M+1)*• at m/z = 137, relative height = 1.2%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 10 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What fragments might you expect in the mass spectra of the following compounds?arrow_forwardWhen measured on a spectrometer operating at 200 MHz, chloroform (CHCl3) shows a single sharp absorption at 7.3 δ. (a) How many parts per million downfield from TMS does chloroform absorb? (b) How many hertz downfield from TMS would chloroform absorb if the measurement were carried out on a spectrometer operating at 360 MHz? (c) What would be the position of the chloroform absorption in δ units when measured on a 360 MHz spectrometer?arrow_forwardFollowing is the mass spectrum of bromocyclopentane. The molecular ion m/z 148 is of such low intensity that it does not appear in this spectrum. Assign structural formulas for the cations of m/z 69 and 41.arrow_forward
- A compound containing only carbon, nitrogen, oxygen, and hydrogen contains four carbon atoms. If the M+ peak in its mass spectrum appears at m/z = 87, then how many nitrogen atoms does it contain?arrow_forwardThe mass spectrum of an aldehyde shows a parent peak at m/z = 58 and a base peak at m/z = 29. Propose a structure, and identify the two species whose m/z values were listed. Name the compound in the box below.arrow_forwardWhich of the following compounds appears in the mass spectrum to have a baseline peak at m/z=43, indicating the reason OH B OH OH C Darrow_forward
- Match each structure to its mass spectrum.arrow_forwardPropose a molecular formula for a compound that exhibits the following peaks in its mass spectrum Problem 1: (M)+• at m/z = 72, relative height = 38.3% of base peak; (M+1)+• at m/z = 73, relative height = 1.7% of base peakarrow_forwardThe mass spectrum of an unknown compound has a molecular ion peak with a relative intensity of 56.11% an an M+1 peak of 5.70%. How many carbon atoms are in the compound? (Fill in an integer number)arrow_forward
- In a mass spectrum, what m/z value would correspond to the fragment shown below?arrow_forwardYou have a unknown compound you need to identify - its El mass spectrum shows a peak at m/z=187 with an intensity of 84, and a peak at m/z = 188 with an intensity of 11. There are no peaks with m/z > 200. What does this %3D mean? The compound contains approximately 30 carbon atoms The compound probably contains a nitrogen atom The compound probably contains a bromine atom Not enough information to tell anythingarrow_forward1. Given the parent compound, chlorobenzene, draw the mass spectrum fragment that is observed at m/z 77. Include any hydrogen atoms and the charge. H H C H H H 2. Given the parent compound, isobutyl-benzene, draw the mass spectrum fragment that is observed at m/z 91. Include any hydrogen atoms and the charge. CH3 CH3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning