
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
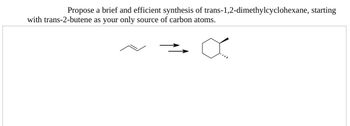
Transcribed Image Text:Propose a brief and efficient synthesis of trans-1,2-dimethylcyclohexane, starting
with trans-2-butene as your only source of carbon atoms.
a
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Provide an IUPAC name for the compound below. ball & stick v + labels (Specify (E) or (Z) stereochemistry, if relevant, for straight chain alkenes only. It is not necessary to use italics in writing compound names Pay attention to commas, dashes, and parentheses. )arrow_forwardSynthesize: OH & its enantiomer Synthesize: 3-methyl-1-pentene From: (E)-3,4-dimethyl-hex-2-ene From: 3-methyl-2-pentanolarrow_forwardWhat is the percentage of HCl in the solution if the molarity is 0.268398000? Assume the density of the solution is 1.00 g/mL.arrow_forward
- Describe a sequence of reactions by which 2-hexyne can be prepared from acetylene while minimizing the number of steps required. O 1. NANH2; 2. CH3CH2CH2Br; 3. NaNH2; 4. CH3B1 O 1. NANH2: 2. CH3Br; 3. CH3CH2CH2B1; O 1. NANH2; CH3Br; 3. NANH2; 4. CH3CH2CH2B O A or B O A or Carrow_forwardChoose the right reagent or series of reagents from the ones listed to synthesize the following molecule (see picture) from benzene (C6H₁) or toluene (C6H5 CH₂). COOH NH₂ C6H₁ and HNO3, H₂SO4 followed by CH3 Cl, AlCl3 then KMnO4, A ○ C₁H₂CH3 and KMnO4, A, followed by HNO3, H₂SO4 ○ C6H₂ CH3 and HNO3, H₂SO4, followed by KMnO4, A, then Zn, HCI C6H₁ and CH3OH followed by KMnO4, A, then HNO3, H₂SO4 C6H₁ CH3 and HNO3, H₂SO4, followed by Zn, HC1arrow_forwardHelp pleasearrow_forward
- the complete hydrogenation of 2,4-octadiyne will result in what product?arrow_forward11.; (a) Similar to alkanes, hydrogen gas can undergo radical bromination according to the reaction below. Propose a chain-reaction mechanism for this reaction, including an initiation step, propagation steps, and two plausible termination steps. The homolytic bond dissociation energy for Br-Br is 46 kcal·mole', for H-Br is 88 kcal'mole and for H-H is 104 kcal'mole'. hv H-H + Br-Br 2 H-Br (b) Calculate the overall AH for the above propagation steps (show all work).arrow_forward(a) In an acid-catalyzed hydration, one of the following 10 carbon alkynes is expected to produce a single hydration product? Select the correct alkyne and draw the structure of the hydration product that is formed from this alkyne. (I) 2-decyne; (II) 3-decyne; (III) 4-decyne; (IV) 5-decyne; (V) none of these will give a single hydration product. (b) The reaction shown below gives Compound X as the major product. The mass spectrum of X is shown. Br2, H20 Compound X 100 - MS-IW-5644 80 60 40 - 20 - 20 40 60 80 100 120 140 160 180 200 220 m/z Considering the reactions of alkynes and the MS data, de duce which of following structures corresponds to X: Br Br HO, IV V I II II Support your answer with a reaction mechanism for fomation of X and identification of relevant peaks in the mass spectrum. 12 Relative Intensityarrow_forward
- How is the mechanism for oxymercuration/demercuration reactions of alkenes understood? How are the (Markovnikov) products predicted and what reagents would you need to use to incorporate this reaction into a multistep synthesis strategy. Finally, what makes this a potentially more useful way to synthesize alcohols from alkenes than simple acid hydration?arrow_forwardPlease draw the structures of (E)-1-chloropropene, 1,1-dichloro-2-methylpropene, and (E)-2,3-dichloro-2-butene and please rank them in terms of increasing dipole moment Arrange the following alkenes in order of increasing stability: 1-pentene; (E)-2-pentene; (Z)-2-pentene; 2-methyl-2-butene, thank youarrow_forwardGive a clear explanation handwritten in detailed of both subparts pleasearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY