
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Suppose a chloride ion and a sodium ion are separated by a center–center distance of 5 Å. Is the interaction energy (the energy required to pull them infinitely far apart) predicted to be larger if the medium between them is water, or if it is n-pentane? If Ca 2+, Na+, and F - each have ionic radii ∙1.16. Which ionic bond is stronger: Ca-F or Na-F? If Ca 2+ is often bound on the surface of a protein by
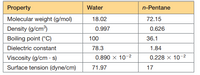
Transcribed Image Text:Property
Water
n-Pentane
Molecular weight (g/mol)
18.02
72.15
Density (g/cm)
0.997
0.626
Boiling point (°C)
100
36.1
Dielectric constant
78.3
1.84
Viscosity (g/cm · s)
0.890 x 10-2
0.228 x 10-2
Surface tension (dyne/cm)
71.97
17
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Based on the bond energies for the reaction below, what is the enthalpy of the reaction? HCECH(g) + 5/2 0:(g)→ 2 CO:(g) + H:O(g) Single Bond 432 |kJ 411 346 N 386 305 167 459 358 201 142 CC 602 799 835 C 0 1072 Multiple Bonds 615 O=0 494 4. 6. 887 N N 942 3. 2.arrow_forward(Born-Haber cycle); Cl appears to undergo deposition but it isn’t given, overall this question just doesn’t add up to me.arrow_forwardList the compounds in order of decreasing covalence and explain why: HI, HCl, HF, HBrarrow_forward
- ding 1.pdf (63.5 KB) AH (kJ) -400 Chemical Equation Process Formation M(s) +1/2X2(g)→MX(s) 55 Sublimation 246 First lonization Energy Bond dissociation 200 -150 X(g) + e (g) → X(g) Electron Affinity Lattice Energy చంత తarrow_forwardLet Su, Tn, and VI be imaginary elements in the same column in the periodic table. Their relative electronegativities are Su > Tn > VI and their relative atomic radii are VI > Tn > Su. Which of the following anions would have the highest K₁? ⒸHSUO₂ ⒸHVIO₂ ⒸHTnO₂ All of these would be expected to have the same Kb.arrow_forwardHow many valence electrons does ns^2 np^4 havearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY