
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
PROBLEM-SOLVING:
The following data shows the decomposition of hydrogen peroxide, H2O2 in an aqueous solution over time.
1. Construct the C vs t plot on a, indicate the proper labels.
2. Determine the order of reaction
3. Calculate:
-Slope of the line
-Rate constant
-Half-life
-Shelf-life
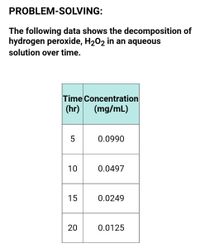
Transcribed Image Text:PROBLEM-SOLVING:
The following data shows the decomposition of
hydrogen peroxide, H202 in an aqueous
solution over time.
Time Concentration
(hr)
(mg/mL)
5
0.0990
10
0.0497
15
0.0249
20
0.0125
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- The rate expression for the reaction between H2O2 and KI is:rate = k[H2O2]m [KI]nwhere: k = rate constantm = order of reaction with respect to H2O2n = order of reaction with respect to KI Table 2: Molarity of H2O2 and KI and Reaction Rate Trial H2O2 Concentration, M KI Concentration, M Reaction Rate(Reciprocal Slope) 1 0.29 M 0.40 M 14.08 2 0.29 M 0.20M 25 3 0.023 M 0.40 M 20 Order with respect to H2O2: -0.138 Order with respect to KI: -0.828 Rate constant value Trial 1: 5.84 Trial 2: 5.55 Trial 3: 5.56 Average: 5.6 -Please help me with this part, please 1.Write the complete rate law for this reaction. 2.List two sources of error for this experiment.arrow_forwardhello.thanksarrow_forward< 11:28 A) Zeroth What order of reaction has a linear curve when plotting concentration versus time? B) First C) Second Question 6 of 12 D) Third Submit E) All reaction orders have a linear curve when plotted Tap here or pull up for additional resourcesarrow_forward
- The rate expression for the reaction between H2O2 and KI is:rate = k[H2O2]m [KI]nwhere: k = rate constantm = order of reaction with respect to H2O2n = order of reaction with respect to KI Table 2: Molarity of H2O2 and KI and Reaction Rate Trial H2O2 Concentration, M KI Concentration, M Reaction Rate(Reciprocal Slope) 1 0.29 M 0.40 M 14.08 2 0.29 M 0.20M 25 3 0.023 M 0.40 M 20 please help me with this part and please show me all steps Rate constant value Trial 1 ______________ Trial 2 ______________ Trial 3 ______________ Average _____________arrow_forwardplz solve it within 30-40 mins I'll upvote your answerarrow_forwardPlease don't provide handwritten solutionarrow_forward
- Please help, thanks.arrow_forwardDetermining Individual Reactant Order and Half-Life from Plotted Data-Your Turn #1 Consider a chemical reaction, where "A" is one of the reactants. During the course of this chemical reaction, the concentration of A was measured as a function of time, and the results were plotted: 1)Analyze these plotted data, complete the 0.9 0.8 table below. 0.7 0.6 2)Work with these [(t)] data and MS Excel to determine the reaction order with respect to the concentration of "A". How are you going to manipulate the above equations and re-plot the tabulated numbers to determine the order? 三 05 30.4 0.3 0.2 0.1 50 100 150 200 250 300 350 400 450 500 3)Finally, calculate the rate constant 'k' using your Excel work and find a way to estimate half-life of "A" (both with proper units). Time (seconds) Order in [A] = k = half-life =.arrow_forwardCalculate the time (min)required for a first-order reaction A -> Product to be 64.9% completed, knowing that the half lifetime t 1/2 is 206 minarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY