
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Looking for net ionic equation
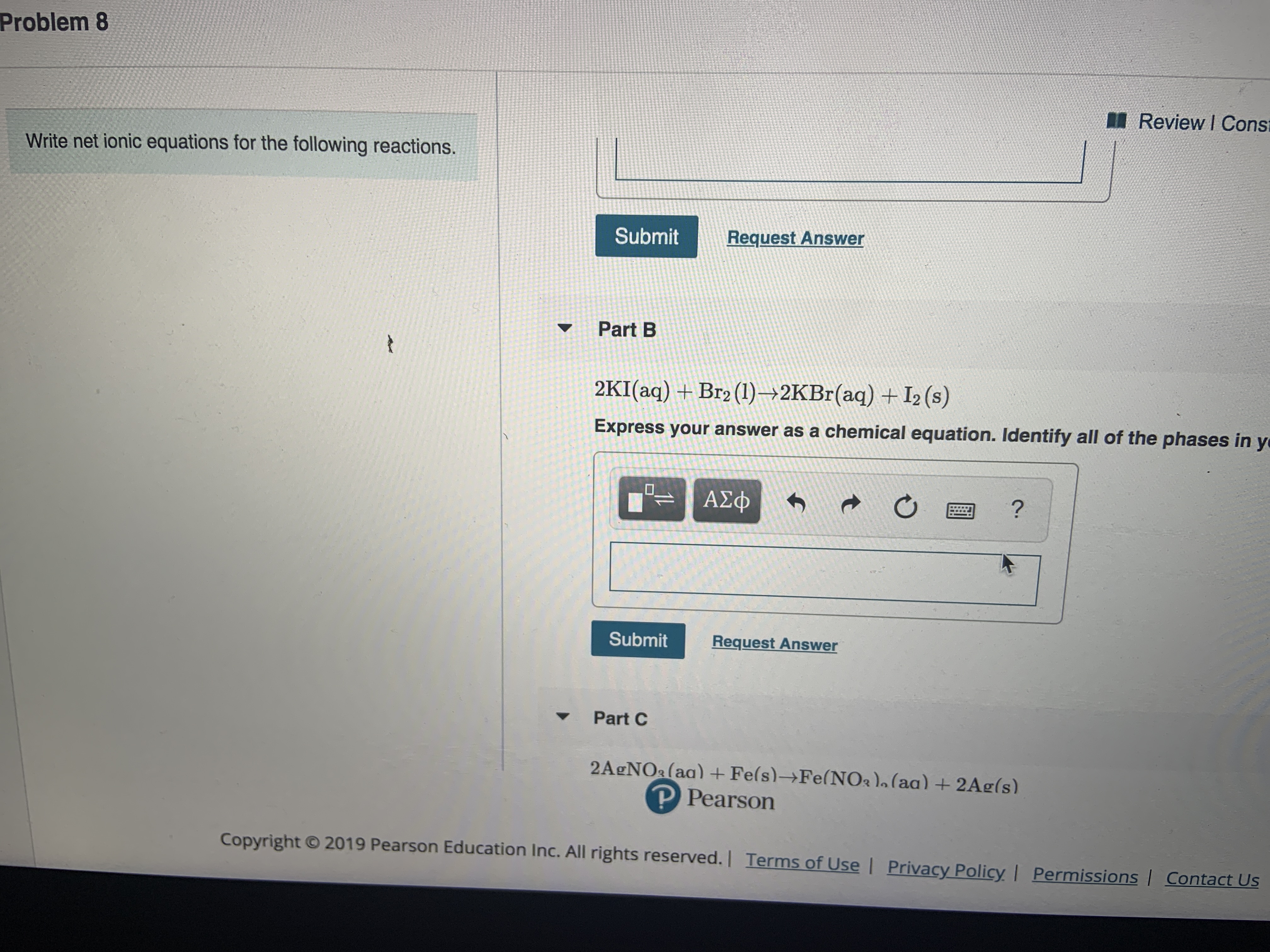
Transcribed Image Text:Problem 8
Review I Cons
Write net ionic equations for the following reactions.
Submit
Request Answer
Part B
et
2KI (aq) +Br2 (1) 2KBr(aq) + I2 (s)
Express your answer as a chemical equation. Identify all of the phases in y
-ΑΣΦ
ΑΣφ
?
Submit
Request Answer
Part C
2A&NO&(aa) +Fe(s)-Fe(NO2)a (aa) +2Ag (s)
P Pearson
Copyright
2019 Pearson Education Inc. All rights reserved.| Terms of Use I Privacy Policy | Permissions | Contact Us
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 7 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Question 2 What is the coefficient of the bromide ion when the following equation is balanced? MnO, +BrMn* +Br, (acidic solution) 3. Question 3 2.arrow_forwardNumber 81. I can write balance equation and part A I did easily. I do not understand how they can't answer for B? and I even looked at the solution book and I don't know how they're adding water as a reaction. Feel free to see oic of solution to get the delta H. the question has three equations, yet the solution Book uses foour: I just don't know how They got answerarrow_forwardIron (iii) chloride + Potassium phosphate Molecular Equation Ionic Equation Net Ionic Equationarrow_forward
- Write "most" under the compound which would be most reactive toward Bry/Feßry. Write "least" under the compound which would be least reactive toward Bry/FeBr). benzene benzoic acid phenol Write "highest" under the compound in the highest oxidation state. Write "lowest" under the one in the lowest oxidation state. CH Write "most" under the one which is most acidic. Write "least" under the one which is least acidic. cyclohexanol benzene OH phenol region.arrow_forwardWhat is you observation when Hydrochloric acid reacts with sodium hydroxide? What color solution they form?arrow_forwardNet lonic Equation Driving Force Copper (II) Sulfate + Sodium Nitrate nothing. Observation Molecular Equation Complete Ionic Equation Net Ionic Equation Driving Force 47arrow_forward
- Write the overall chemical equation, the complete ionic equation, and the net ionic equation for the reaction of aqueous silver fluoride with aqueous sodium phosphate to give solid silver phosphate and a solution of sodium fluoride. Answer:arrow_forwardAqueous hydrobromic acid is added to a beaker containing solid calcium carbonate. How could the aqueous product here be distinguished from the aqueous product formed if strontium carbonate were substituted for calcium carbonate? i) Balanced equation: ii) Answer:arrow_forward3. Write the balanced chemical equation, total ionic equation, net ionic equation and spectator ions for each of the following: a) Sodium chloride and lead (II) nitrate b) Sodium phosphate and nickel (II) perchloratearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY