
Understanding Motor Controls
4th Edition
ISBN: 9781337798686
Author: Stephen L. Herman
Publisher: Delmar Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
This problem is not part of a graded assignment. Solve this problem and show all of the work
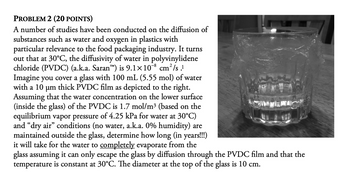
Transcribed Image Text:PROBLEM 2 (20 POINTS)
A number of studies have been conducted on the diffusion of
substances such as water and oxygen in plastics with
particular relevance to the food packaging industry. It turns
out that at 30°C, the diffusivity of water in polyvinylidene
chloride (PVDC) (a.k.a. Saran™) is 9.1×108 cm²/s.1
Imagine you cover a glass with 100 mL (5.55 mol) of water
with a 10 μm thick PVDC film as depicted to the right.
Assuming that the water concentration on the lower surface
(inside the glass) of the PVDC is 1.7 mol/m³ (based on the
equilibrium vapor pressure of 4.25 kPa for water at 30°C)
and "dry air" conditions (no water, a.k.a. 0% humidity) are
maintained outside the glass, determine how long (in years!!!)
it will take for the water to completely evaporate from the
glass assuming it can only escape the glass by diffusion through the PVDC film and that the
temperature is constant at 30°C. The diameter at the top of the glass is 10 cm.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- please solve part d and earrow_forwardA student working 3.0m away from you in a chemistry lab performs an experiment which creates a bad odor. It takes a while for the smell to get to you (air currents negligible). You are able to move 2.0m further away from the source but the smell catches up with you in another 60 seconds. What is the diffusion coefficient in the air for the molecules you smell?arrow_forward10:52 AM 50 SALE Noll 2.0376 K/S Downloaded by Mothusi Mahlathini (mothusimothusi194@gmail.com) SECTION A Question 1 (a) (b) (i) List and briefly explain the four components of the discipline of materials science and engineering in a linear relationship. [4] (ii) Using a flowchart, classify engineering materials, indicating the types of polymers. [5] The ionic radii and coordination number of NaCl is 0.414 -0.732 and 6 respectively. Using the data below, show that FeO has the structure of NaCl. [8] (c) Ion Ionic radius (cation/anion) (nm) Al3+ 0.053 Fe3+ 0.069 02- 0.140 CI- 0.181 F- 0.133 Fe2+ 0.077 Determine the Miller indices for the directions in the cubic unit cell shown below (Fig. Q1): [8] Figure Q1 Downloaded by Mothusi Mahlathini (mothusimothusi194@gmail.com) Question 2 (a) (b) T (i) List and briefly explain the six properties of solid materials. [6] (ii) Using a flowchart, classify metals and alloys. [6] BCC lithium has a lattice parameter of 3.5089 x 10-10 m and contains 200…arrow_forward
- Sodium chloride (NaCI) is a ceramic that has the following crystal structure. Given: R(Na*)= 0.098 nm and R(CI")=0.181 Calculate the lonic Packing Factor (IPF) of NaCl? O Na CH Select one: O a. 0.14 O b. 0.66 C. 0.51 O d. 0.74 O e. 0.41 O f. 0.47 g. 0.21arrow_forwardOnly questions 4,5,6 I need help witharrow_forwardAnswer whats in the image, please and thank youarrow_forward
- Design and manufacture of plastic and composite parts The tank shown in Figure 1 is stacked as follows: Couches individuelles: verre = glass 1 - Unidirectionnel verre/epoxy, V₁ = 62%, h = 0.010 po. 2- Mat verre/epoxy, V₁ = 40%, h = 0.040 po. Extérieur 4 Empilement: [0/35/-35/90] 3 2 3 1 Intérieur Unidirectionnel kevlar-epoxy, V₁ = 55%, h = 0.010 po. 4- Tissus 50/50, Kevlar/epoxy, 230 g/m2, V₁ = 50% h = épaisseur du PLI V₁ = Pourcentage volumique de fibres As well as the following load and dimensions: P = 3 MPa r = 50 mm L = 625 mm h = 600 mm F = 500 N N h р B x F Figure 1. multilayer composite pipe under pressure (p), also subject to torsional moment and bending moment due to force F and lever arms h and L. page 1arrow_forwardThe Occupational Safety and Health Administration has set a Permissible Exposure Limit of 1000 parts per million (ppm) of acetone. You are an industrial hygienist doing personal sampling on a worker who is supervising a production process that uses acetone. The upcoming exposures can be measured: 700 ppm for three hours and 1300 ppm for two hours. 3 hours at 900 ppm What is the TWA as calculated? What advice would you provide plant management regarding acetone exposure and PEL adherence?arrow_forwardThe major difference between solid and fluid is the molecules are well-packed in solid but free to move in fluid. As a consequence of such difference, which of the following statements are correct? (i) Stress is proportional to strain in solid but stress is proportional to rate of strain in fluid. (ii) Fluid can be used as a lubricating film to separate 2 solid surfaces from direct contact to reduce friction. (iii) The energy required to move a ship slowly is low but increases rapidly with speed. (A) (B) (i) and (ii) (i) and (iii) (ii) and (iii) All of the abovearrow_forward
- Nylon 6,6 is a thermoplastic polymer with high mechanical strength, high resistance to chemical environments, and good stability under heat. Therefore, it is well suited to be used in 3D structural objects. A cylindrical nylon 6,6 pipe is made by injection molding. The pipe is 1 m long with an outer diameter of 8cm, and a thickness of 2mm. The crystallinity of the pipe is 25%. The density and associated percent crystallinity for two nylon 6,6 samples are measured as below: ρ(g/cm3) crystallinity (%) 1.190 67 1.155 44 Calculate the density of this pipe, which has 25% crystallinity.arrow_forwardNylon 6,6 is a thermoplastic polymer with high mechanical strength, high resistance to chemical environments, and good stability under heat. Therefore, it is well suited to be used in 3D structural objects. A cylindrical nylon 6,6 pipe is made by injection molding. The pipe is 1 m long with an outer diameter of 8cm, and a thickness of 2mm. The crystallinity of the pipe is 25%. The density and associated percent crystallinity for two nylon 6,6 samples are measured as below: ρ(g/cm3) crystallinity (%) 1.190 67 1.155 44 If the degree of polymerization is 500, calculate the number of chains in the pipe. (Assume the chains are equal in length).arrow_forwardKindly solve it completely by hand if possible.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Understanding Motor ControlsMechanical EngineeringISBN:9781337798686Author:Stephen L. HermanPublisher:Delmar Cengage Learning
Understanding Motor ControlsMechanical EngineeringISBN:9781337798686Author:Stephen L. HermanPublisher:Delmar Cengage Learning

Understanding Motor Controls
Mechanical Engineering
ISBN:9781337798686
Author:Stephen L. Herman
Publisher:Delmar Cengage Learning