
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
a.) Calculate the magnitude of Qc, the amount of heat lost in cooling.
b.) Calculate the work done during compression (a-b on the p-v diagram).
c.) Calculate the work done during combustion (b-c on the p-v diagram
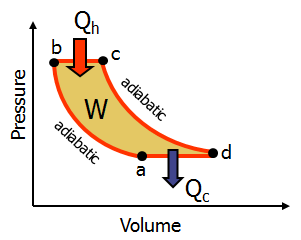
Transcribed Image Text:Pressure
Qh
W
adiabatic
adiabatic
a
Volume
Qc
d
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Match each of the terms given below with the particular constraints that it implies about a thermodynamic process. isothermal isobaric A. dq = 0 Β. ΔΡ = 0 C. dw = 0 D. AV = 0 isochoric ◆adiabatic E. An = 0 F. AT=0arrow_forwardThe density of ice and water are 917 kg/m³ and 1000 kg/m³. The latent heat for the transition between ice and water is 334 kJ/kg. Calculate the slope of the tangent of the coexistence curve of ice and water at T = 213 K. Select one: dP dT a. b. C. O d. dP dT dP dT dP dT = - -170 atm/K = — - - 181 atm/K -203 Pa/K -183 Pa/Karrow_forwardWhich of the following is true about an adiabatic process? Select all those apply. I. It occurs at a constant temperature (AT =0). II. There is no transfer/ exchange of heat (Q = 0) in this process. III. The pressure remains constant. IV. The volume remains constant. V. The adiabatic curve is steeper than the isothermal curve in PV diagram. VI. The isothermal curve is steeper than the adiabatic curve in PV diagram. Answer choices: A. I, II, and V B. I, II, and V C. I, III, and IV D. Il and V E. Il and VIarrow_forward
- 5. For a system, the thermodynamic as)vn energy U is defined as a function of S, V, and n which is U(S,V,n) = kn3V 3e3nR %3D where S is the entropy, V is the volume, n is the number of moles, K is the constant, and R is the gas constant. Determine a. Based on the theory in Thermodynamics, (), T = Determine, b. Based on the theory in Thermodynamics, Pressure P = . S,n 5/8 Determine c. Determine ne d. Determine dU in dS, dV and dnarrow_forwardView Policies Current Attempt in Progress Air is compressed in a piston-cylinder assembly from p₁ = 25 lbf/in², T₁ = 500°R, V₁ = 9 ft³ to a final volume of V₂ = 1 ft3 in a process described by pv¹.25 = constant. Assume ideal gas behavior and neglect kinetic and potential energy effects. Using constant specific heats evaluated at T₁, determine the work and the heat transfer, in Btu.arrow_forward2 What is the thermal efficiency for the heat engine illustrated on the pV diagram below? The gas is monatomic p(Pa) 10 V(m³) 7 3arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON