
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Predict the structure(s) of the major organic product(s) that will form when the organic starting material given is subjected to the indicated reaction conditions:
Show stereochemistry clearly using normal bonds, wedge bonds, and dashed wedge bonds as needed. If no reaction occurs for the indicated conditions, write no reaction as your answer.
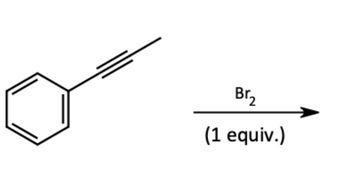
Transcribed Image Text:Br₂
(1 equiv.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- I'm not sure how to go about calculating for percent yield of my product. Do I need to calculate theoretic yield first? I always mix up the formulas. I was performing the horner wadsworth reaction , synthesis of 3,4-Methylenedioxystilbene. The reaction was between diethyl benzylphophonate and 3,4-dimethylenedioxybenzaldehyde. Aliquat 366 was the catalyst.arrow_forwardClaisen Condensation: 7.50 grams of methyl acetate is reacted with water and sulfuric acid catalyst under heated conditions. What amount of water (in grams) is required for the reaction and what is the theoretical yield of the acetic acid product? Include a balanced equation.arrow_forwardPredict the structure of the major organic product that contains a carbonyl group that will form when the organic starting material is subjected to the indicated reaction conditions. If no reaction occurs, indicate that fact in the answer file that you submit. It is not necessary to provide structures or formulas for any inorganic byproducts that also form in the reaction. 1) I, (excess), NaOH 2) H,o* CARBONYL COMPOUND Your structural aarrow_forward
- For each of the following , supply a structure for the major organic product when the product is not given; if no reaction occurs, write NO REACTION . Provide the inorganic reactant (reagent ) or catalyst is missing, simply give a formulaIf heat and / or light is needed, be sure to indicate it appropriately if these conditions are missingarrow_forwardQuestion:In organic chemistry, the formation of carbocations is a critical step in many reactions. Consider the following scenario: A tertiary alcohol, 2-methyl-2-butanol, undergoes acid-catalyzed dehydration to form an alkene. However, when this reaction is attempted using concentrated sulfuric acid, an unexpected product is obtained. Explain the factors responsible for this unexpected product formation and propose a mechanism for the reaction.arrow_forwardWhich of the following statements is incorrect? O One product of the ozonolysis of 1-butyne is carbon dioxide. O Hydration of 2-butyne would give one unique ketone as a product. O The hydrogenation of 1-butene releases less heat than the hydrogenation of trans-2-butene. The epoxidation of an alkene using MCPBA is stereospecific. A common source of carbon dioxide is dry ice.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY