
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
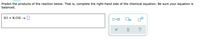
Transcribed Image Text:Predict the products of the reaction below. That is, complete the right-hand side of the chemical equation. Be sure your equation is
balanced.
HІ + КОН
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Use the following information to answer this question. 2Al) + 3CuCl2(aq)→ 2AICI3(aq) + 3Cu(s) (word equation: aluminum plus three copper(Il) chloride produce two aluminum chloride and three copper) Which of the following best describes what is occurring during this reaction? Select one: O a. Aluminum replaces chlorine in the compound. O b. Aluminum replaces copper in the compound. O c. Copper replaces aluminum in the compound. O d. Chlorine replaces copper in the compound. Smoke Signal.pdf EFiles (1).zip Chap6 SF10 (1).pdfarrow_forwardPredict the products of the following reaction. If no reaction will occur, use the NO REACTION button. Be sure your chemical equation is balanced! Caco, () + HBr(aq) → 0arrow_forwardBalance the chemical equation below. Be(C2H3O2)2 + Al2(CO3)3 → BeCO3 + Al(C2H3O2)3 What type of reaction is this?arrow_forward
- How do you balance this equation: NH4OH + KAl(SO4)2*12H2O Please submit detailed step by step on how to balance this equation.arrow_forwardIdentify the type of each of the following reactions. 1. N2(g)+2O2(g)→2NO2(g) -- 2. CaCl2(aq)+2AgNO3(aq)→2AgCl(s)+Ca(NO3)2(aq) -- 3 .CS2(l)+3O2(g)→2SO2(g)+CO2(g) -- 4. H3PO4(aq)+3KOH(aq)→3H2O(l)+K3PO4(aq)-- options for each : --precipitation, acid-base, oxidation-reduction, or no reactionarrow_forwardPlease don't provide handwritten solutionarrow_forward
- The products of the reaction Ba(OH) 2 + H 2SO 4 → arearrow_forwardPredict the products of the reaction below. That is, complete the right-hand side of the chemical equation. Be sure your equation is balanced. HI + NaOH → I Don't Know 1841 Submit 3 E 5 T 6 G 0-0 X S stv♫ 5 © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Cer A all 9 zoomarrow_forwardNot gradedarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY