
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
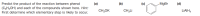
Transcribed Image Text:Predict the product of the reaction between phenol
(a)
(Б)
(c)
MgBr
(d)
(C6H5OH) and each of the compounds shown here. Hint:
First determine which elementary step is likely to occur.
CH;OK
CH;Li
LIAIH,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- 6. Which of the following will not give Hoffmann bromamide reaction ? (а) CH3 - С-NH2 (Ъ) Ph — С-NH, || (с) CН3 - С-NH - Br (d) Ph – C– NH – Pharrow_forwardb) Provide a detailed mechanism (with arrows) and show all relevant reaction intermediates for the following azo coupling reaction. -ZEZ Br NO CIO N Br 'N-arrow_forward3) Any compound or ion with at least one lone pair can be considered a base and/or a nucleophile. The relative basicity and nucleophilicity of the species is one of the most important factors that determine the mechanism of a reaction on sp³-carbon atoms. Please show an example of a) a reagent that is a strong nucleophile and a strong base, b) a reagent that is a weak nucleophile and a strong base, c) a reagent that is a strong nucleophile and a weak base, d) a reagent that is a weak nucleophile and a weak base. Which nitrogen atom in the structure below is most nucleophilic? Please explain by discussing the electron density around each nitrogen atom. Show at least three resonance structures of the compound. N.arrow_forward
- Show the mechanism of the reaction of drawing 1 mole of water from the 2,2,5-trimethyl-3-hexanol compound, indicating the reaction conditions, step by step. Indicate the main product and by-product. b) Does the main product show the geometric isomer? If it does not show the isomers, please indicate why it does not. c) Write the products formed when the. main product ozonlamp is reduced.arrow_forward5. Provide the product that will result for the following reaction and provide the complete mechanism. CH,N2 НО 오arrow_forwardWhich of the following substrates can undergo an E2 step with H2N¯ as the base? For those that can, draw the curved arrow notation and the products. (a) (b) CI (c) (d) (e) `Br Brarrow_forward
- Determine which mechanism below is the most correct. Be sure to look at whether the most acidic H is reacting, if the direction of the arrows are correct, and if the correct products are formed based on the mechanism. If the correct answer is Option B, then explain why the other options are incorrect? Explain each option that is incorrect individually.arrow_forward4. The following reaction results in the formation of not one, not two, but six(!) different compounds. In short, it is a tragic mess, but a good one for us to study. Propose a mechanism for the formation of each product. HINT: think about different possible resonance forms of the reactive intermediate, and recall that stereoisomers are different compounds. Br. Ph H₂O 6 productsarrow_forwardhelp me pleasearrow_forward
- What is/are the product(s) of the reaction of 2-bromopropane with NaOCOCH3? Draw the mechanism & identify it as Sn1, Sn2, E1, or E2. Please explain your reasoning.arrow_forwardCompound A reacts with B in the presence of sodium hydride to give C as the majority product.1. Give the detailed mechanism of the formation of C and draw the product C in the box above.2. Draw the energy diagram of the reaction. Indicate with an arrow the rate-determining step of the reaction then draw its transition state3.The rate of the reaction observed is 5 x 10-2 M.s-1 when the concentrations of A and B are 0.2 M and 0.1 M respectively.Determine the rate of the reaction if the concentrations of A and B are now 0.3 M and 0.2 M respectively. Show your calculationarrow_forwardPlease don't provide handwritten solution ....arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY