
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
:$:$:$;$$:$$:$
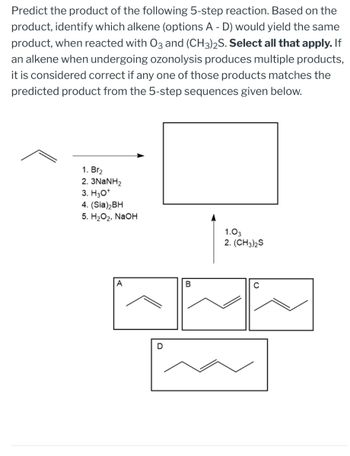
Transcribed Image Text:Predict the product of the following 5-step reaction. Based on the
product, identify which alkene (options A - D) would yield the same
product, when reacted with 03 and (CH3)2S. Select all that apply. If
an alkene when undergoing ozonolysis produces multiple products,
it is considered correct if any one of those products matches the
predicted product from the 5-step sequences given below.
1. Br2
2. 3NaNH2
3. H3O+
4. (Sia)2BH
5. H2O2, NaOH
1.03
2. (CH3)2S
A
B
C
D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 114.9 kj into kilocaloriesarrow_forwardOnce he executes the daring jump out of the plane the skydivers energy is converted to what type of energy?arrow_forward1. Water has a specific heat of 4.184 J/g°C while glass (Pyrex) has a specific heat of 0.780 J/g °C. If 10.0 J of heat is added to 1.00 g of each of these, which will experience the larger increase of temperature? a. Glass b. Water a b d c. They both will experience the same change in temperature since only the amount of a substance relates to the increase in temperature. d. There is not enough information provided to answer this questionarrow_forward
- The specific heat of iron is 0.108 cal/g °C. If 665 cal of heat is added to 414.0 g of iron at 25.0 °C, what is the new temperature of the iron?arrow_forwardConvert the heat of neutralization of acetic acid from -48.6 kj/mmol to calories per millimole and ROUND TO THE NEAREST WHOLE NUMBER AND INCLUDE THE APPROPRIATE SIGN (1 cal = 4.184 J) DO NOT INCLUDE UNITS Type your answer...arrow_forwardA chunk of brass is initially at 25°C. The brass absorbs 75 J of heat and the temperature increases to 45°C. What size is the chunk of brass (in grams)? The specific heat of brass is 0.38 J'g-1.°C-1. A. 9.9 g brass B. 3.8 g brass C. 570 g brass D. 1.4 g brassarrow_forward
- Convert 76 kcal to kJarrow_forwardConvert 51.7 calories to Joules.Answer in units of J.arrow_forwardCopper wires used to transport electrical current heat up because of the resistance in the wire. If a 136-g wire gains 251 J of heat, what is the change in temperature of the wire? Copper has a specific heat of 0.384 Jg-l °C-1. Change in temperature =| °Carrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY