
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
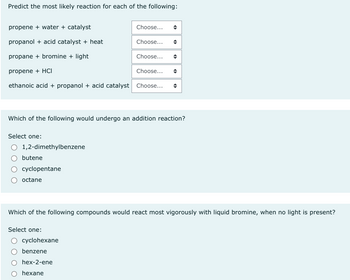
Transcribed Image Text:Predict the most likely reaction for each of the following:
propene + water + catalyst
propanol + acid catalyst + heat
propane + bromine + light
propene + HCI
ethanoic acid + propanol + acid catalyst Choose...
Select one:
1,2-dimethylbenzene
butene
cyclopentane
octane
Choose...
Which of the following would undergo an addition reaction?
Select one:
Choose...
cyclohexane
benzene
hex-2-ene
hexane
Choose...
Choose...
(
Which of the following compounds would react most vigorously with liquid bromine, when no light is present?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 8 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The respective Heats of Hydrogenation for three unkown alkenes are determined to be: 118.7 kJ/mol 124.5 kJ/mol 128.0 kJ/mol We have 3 possible structures for these alkenes, as shown. Given the structures shown, which of the following statements is most likely true? Compound A Compound B Compound C The Heat of Hydrogenation for Compound B is 118.7 k/mol, (AND) The Heat of Hydrogenation for Compound A is 128.0 kJ/mol The Heat of Hydrogenation for Compound A is 118.7 kJ/mol (AND) The Heat of Hydrogenation for Compound C is 128.0 kJ/mol O The Heat of Hydrogenation for Compound C is 118.7 kJ/mol O The Heat of Hydrogenation for Compound B is 118.7 k/mol O The Heat of Hydrogenation for Compound A is 118.7 kJ/molarrow_forwardtudent Name: 7. Draw a bond-line structure of based on the following name. Determine if the systematic name provided is 9600 correct or incorrect. If the name is incorrect, provide a correct systematic name for the molecules. a. 2,2-dimethyl-4-ethylheptane XX nalda b. 5-butyl-3,3,9-trimethylundecane C. 3-isopropyloctane a 4-ethyl-2,2-dimethylheptane Lovel مین آماده همرا nchs husits, no no band a d. 1-methyl-3-sec-butylcyclohexane usu enollsmolno? $ tall at to do thought off والا بس الي الليل with amet gniwollot rose wala er to be anled died J-J planting * * bazgib S dan dras s thot ماء لامعة بومادر to meet to be as la actres des of Rank the alkanes in each group below in order of increasing boiling point (3 = lowest, 1 = highest). do not touted toarrow_forward8. How many different organic products are possible when cyclobutanol is heated in the presence of acid? Ignore any stereoisomers (i.e. cis/trans isomers). Question 8 options: two alkanes one alkane two alkenes one alkenearrow_forward
- which of the following pairs. of compounds represent constitutional isomers? A) 2, 2-dimethyl propane & 2-methylpentane B) 2, 2-dimethy I butane & 3- methylpentane (c) 2, Methyl butane & 2-methylpropane D) All of the abovearrow_forwardMolecular Models in Dichloropropane, Butane,Chlorobutane,Dichlorocyclopropane,Dichloroethenearrow_forwardPlease help me with this List practical and industrial uses of both alcohols and ethers in organic chemistry (Make a charge) also list out environment impacts for both alcarrow_forward
- Which of the following is true? Choose 1. a. Both alkane and alkenes can undergo radical mechanized reaction but mostly for alkanes. b. All reactions of alkenes and alkynes are classified as electrophilic addition reaction. c. Both alkane and alkenes can undergo oxidation reactions to form various oxidation product.arrow_forwardWhich alkenes exist as pairs of cis,trans isomers? For each that does, draw the trans isomer. Q.) CH3CH=CHBrarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY