
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
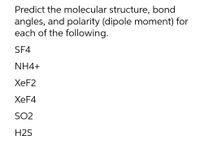
Transcribed Image Text:Predict the molecular structure, bond
angles, and polarity (dipole moment) for
each of the following.
SF4
NH4+
ХeF2
ХeF4
SO2
H2S
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2arrow_forwardChemical formula CH₂Cl₂ NC13 H₂O HCN CH₂0 Lewis structure : Cl H C-H : Cl: How to draw a lowic struct Bonding groups around central atom 4 Lone pairs around central atom 0 Electron pair geometry tetrahedral Molecular geometry 3D structurearrow_forwardDetermine the molecular geometry and molecular polarity for the molecule SiO2. Linear polar Trigonal planar nonpolar Tetrahedral ionic Trigonal pyramidal nonpolar Bent polar Trigonal planar ionic linear nonpolar Tetrahedral polar Bent ionic Tetrahedral nonpolar Trigonal pyramidal polar Trigonal pyramidal ionic Trigonal planar polar Linear ionic Bent nonpolararrow_forward
- 7.Draw the Lewis structure first, then predict the molecular shape and electron group geometry for each of the following: CS2 H3O+ PBr3 SiCl4arrow_forwardWhich statement about the bond angles in the following triatomic molecules is correct? H₂S XeF2 H₂O XeF2 has the largest bond angle The bond angles are all equal H₂O has the largest bond angle H₂S has the largest bond anglearrow_forwardWhich of the following has the largest bond angle? SO3 O NO2 O HCN H2CO3 (the bond angle around the carbon atom) PH3arrow_forward
- Which of the following molecules contains a triple bond? * O NF3 SO2 O C2H4 O co How many lone pairs (non-bonding pairs) are around the central atom (S) in O Nonearrow_forwardWhich of the following is nonpolar? O NO2 SO3 PH3 HCN CHCI3arrow_forwardWhich of the following molecules has 1 lone pair around central atom? SO2 H2O CO2arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY