
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
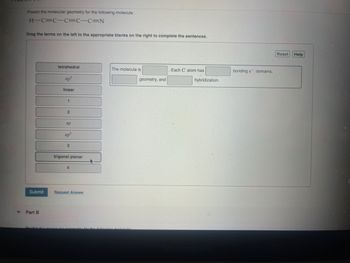
Transcribed Image Text:Predict the molecular geometry for the following molecule:
H-C=CC=C-C=N
Drag the terms on the left to the appropriate blanks on the right to complete the sentences.
tetrahedral
The molecule is
Each C atom has
bonding e domains,
sp³
linear
geometry, and
hybridization.
2
sp
sp²
3
trigonal planar
Submit
Request Answer
Part B
Predict the
for the following molecule
MacBook Pro
Reset
Help
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- 7) Below is the Lewis structure for diethyl ether. Answer the following questions about this structure. a) Are there any polar bonds in this molecule? If yes, label the atoms in the polar bonds with &+ and 8- accordingly. H. H. YES NO H FC H- b) Is the molecule polar? Briefly (1 sentence, max) give your reasoning for your decision. H YES NO :o:arrow_forwardF3 Use the VSEPR model to predict the bond angles about each numbered atom. H H the 1 2 3.. H H Mastered The predicted angles about atom 1 are The predicted angles about atom 2 are The predicted angles about atom 3 are H Submit Answer $ 4 000 % 5 H F5 degrees. Retry Entire Group 9 more group attempts remaining F6 degrees. Cengage Learning Cengage Technical Support & 7 degrees. F7 * 8 if needed for this q DII F8 F9 F10arrow_forwardPart A Predict whether each of the following molecules is polar or nonpolar. Drag the appropriate items to their respective bins. Polar Submit SeF4 NH3 Request Answer GaH3 CH,I XeF 11 Nonpolar CBTA Reset Help Pearsonarrow_forward
- Polar or Electron Pair Shape Molecular Shape Approximate Bond Angles Dot Structure Bonding Sketch Molecule Non-polar CIF3 3- POA IF5 clo, Cs2 XeF 4 C2H;Br Experiment 12 Report Sheet (continued) 121arrow_forward‘Arrange in order of increasing carbon-oxygen bond length’arrow_forwardCategorize each diatomic molecule as diamagnetic or paramagnetic Dlagmagnetic Possible answers B D 02 A A No Answers Chosen ⠀ C N₂ B Paramagnetic F₂ C No Answers Chosen Liz Darrow_forward
- esc ! :8: F1 @ Draw the Lewis structure of AICI, and then determine the electron domain and molecular geometries of the central atom. Click to draw a new structure F2 + # 80 F3 $ 900 000 F4 % F5 MacBook Air F6 & 7 A) trigonal / planar B) trigonal/tetrahedral C) trigonal planar / tetrahedral D) trigonal / bent (120°) E) trigonal planar / trigonal planar Ad F7 * 8 100 DII F8 ( 9 F9 F10 F11 ) + E AA 0 =arrow_forwardTu Please don't provide the handwriting solutionarrow_forwardCHEM 181 Experiment On Central atom Valence Bonding e Domains Molecular Geometry Lewis Dot Structure lone e VSEPR Formula VSEPR Molecules er lon Shell e Domains Pairs Geometry SF4 CIFs CЬ НСо CO2 Н.: C2H2 CHFarrow_forward
- I need help with my homework questionsarrow_forwardN2 14 What are the angles a and b in the actual molecule of which this is a Lewis structure? H EN: Note for advanced students: give the ideal angles, and don't worry about small differences from the ideal that might be caused by the fact that different electron groups may have slightly different sizes. a = b = ]° Explanation Check O 2021 McGraw-Hill Education. All Rights Reserved. Terms of UseI Privacy Accessibility Activity Details 19 MacBook Air 24 30 888 esc FS F1 F2 @ 23 2$ % & 8. 9. 1 W E R Y. U tab D G caps lockarrow_forwardNeed help with all parts of the question.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY