
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Predict the major and minor products.
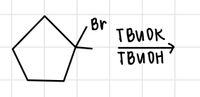
Transcribed Image Text:The image depicts a chemical reaction involving a brominated cyclopentane compound. The chemical structure shows a five-membered carbon ring (cyclopentane) with a bromine (Br) atom attached to one of the carbon atoms, indicating an alkyl bromide.
Above the reaction arrow, "TBuOK" is written, which represents the reagent tert-butoxide, a strong base. Below the arrow, "TBuOH" is noted, indicating tert-butanol as the solvent or by-product.
This setup suggests an elimination reaction, possibly an E2 mechanism, where the base (TBuOK) abstracts a proton from the carbon atom adjacent to the one bearing the bromine atom. This results in the formation of a double bond and the elimination of HBr, producing an alkene.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Rank in order of Increasing Bronsted acidity the structures I, II and III.arrow_forwardFor the following acid-base reaction, predict which side of the equilibrium is favored. O Right-hand side O Left-hand side O Both are alkane protons, so neither side is favored.arrow_forwardI need help with 3b and 3c with an explanation.arrow_forward
- 3. For the following set of compounds, box the most basic and circle the least basic.arrow_forwardWrite "most" under the compound which would be most reactive toward Bry/Feßry. Write "least" under the compound which would be least reactive toward Bry/FeBr). benzene benzoic acid phenol Write "highest" under the compound in the highest oxidation state. Write "lowest" under the one in the lowest oxidation state. CH Write "most" under the one which is most acidic. Write "least" under the one which is least acidic. cyclohexanol benzene OH phenol region.arrow_forwardPredict the major product of the following reaction...arrow_forward
- To the presence of hexane HBr addition PEROXIDE leads to the output: subject to O Citzv base Opposite of Marconikov's rule ● Marconikov basearrow_forward4&6 are attached questions, thank you!arrow_forwardNAOH H2NNH2 5) 4) 3) Excess CH3OH H3O* РСС Br START HERE 1) Mg 2) CH3CHO 1) 2) 6)? Br KMNO4 DIBAL, 7) 8) H3C SOC22 Ag20 NH4OH Ph3P:CHCH2CH3 9) 14) 15) Benzene AlCl3 03 DMS C2H5OH, H3O* 10) 16) 17) HCN 11) H3O* 12) NH3 13)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY