
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
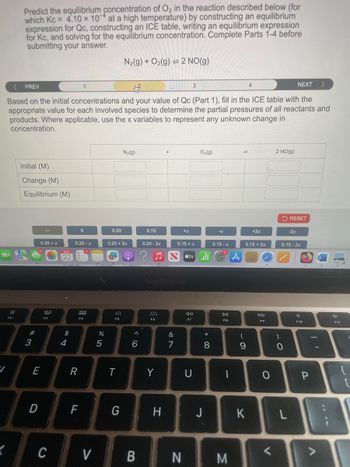
Transcribed Image Text:Predict the equilibrium concentration of O2 in the reaction described below (for
which Kc = 4.10 x 10 at a high temperature) by constructing an equilibrium
expression for Qc, constructing an ICE table, writing an equilibrium expression
for Kc, and solving for the equilibrium concentration. Complete Parts 1-4 before
submitting your answer.
N2(g) + O2(g) 2 NO(g)
<
PREV
3
NEXT
>
Based on the initial concentrations and your value of Qc (Part 1), fill in the ICE table with the
appropriate value for each involved species to determine the partial pressures of all reactants and
products. Where applicable, use the x variables to represent any unknown change in
concentration.
Initial (M)
Change (M)
Equilibrium (M)
N₂(g)
O2(g)
14
2 NO(g)
RESET
0
0.20
0.15
+x
-x
+2x
-2x
0.20 + x
0.20-x
0.20 + 2x
0.20-2x
0.15 + x
0.15-x
0.15+2x
0.15-2x
22
A
A
80
F2
F3
$
54
#3
E
888
F4
F5
%
905
^
6
MacBook Air
---
<<
DII
F7
F8
&
87
R
T
Y
U
F9
* ∞
(
8
9
0
-
C
J
F10
3
D
F
G
H
J
K
L
C
V
B
N
M
V
יו
P
{
V
![Predict the equilibrium concentration of O2 in the reaction described below (for
which Kc = 4.10 x 10 at a high temperature) by constructing an equilibrium
expression for Qc, constructing an ICE table, writing an equilibrium expression
for Kc, and solving for the equilibrium concentration. Complete Parts 1-4 before
submitting your answer.
N2(g) + O2(g) 2 NO(g)
2
3
4
NEXT >
In a 1.0 L container at high temperature, 0.20 mol N2 and 0.15 mol O2 are allowed to react. Set
up the expression for Qc. Each reaction participant must be represented by one tile. Do not
combine terms.
Once the expression is constructed, solve for Qc to determine the direction of the reaction.
Qc =
=
RESET
[0]
[1.0]
[0]²
[1.02
[0.20]
[0.15]
[0.40]
[0.30]
[0.050]
[0.20]²
[0.15]²
[0.40]²
[0.30]²
[0.050]²
0
33
0.030
9.0 × 10-4
1.1 x 104
F2
80
F3
MAR
22
<tv
A
MacBook Air
F5
A
F6
%
6
&
7
0105
694
#3
E
R
T
Y
D
,
<<
DII
DD
F7
F8
F9
U
8
* 0
-
9
61
)
C
O
FAD
F10
LL
F
G
H
J
K
L
I
יו
P
F11](https://content.bartleby.com/qna-images/question/42021111-4ced-4c6f-84e9-5b1969157ec2/164436c5-edc2-4c1f-a112-84e8492ed912/z32fotg_thumbnail.jpeg)
Transcribed Image Text:Predict the equilibrium concentration of O2 in the reaction described below (for
which Kc = 4.10 x 10 at a high temperature) by constructing an equilibrium
expression for Qc, constructing an ICE table, writing an equilibrium expression
for Kc, and solving for the equilibrium concentration. Complete Parts 1-4 before
submitting your answer.
N2(g) + O2(g) 2 NO(g)
2
3
4
NEXT >
In a 1.0 L container at high temperature, 0.20 mol N2 and 0.15 mol O2 are allowed to react. Set
up the expression for Qc. Each reaction participant must be represented by one tile. Do not
combine terms.
Once the expression is constructed, solve for Qc to determine the direction of the reaction.
Qc =
=
RESET
[0]
[1.0]
[0]²
[1.02
[0.20]
[0.15]
[0.40]
[0.30]
[0.050]
[0.20]²
[0.15]²
[0.40]²
[0.30]²
[0.050]²
0
33
0.030
9.0 × 10-4
1.1 x 104
F2
80
F3
MAR
22
<tv
A
MacBook Air
F5
A
F6
%
6
&
7
0105
694
#3
E
R
T
Y
D
,
<<
DII
DD
F7
F8
F9
U
8
* 0
-
9
61
)
C
O
FAD
F10
LL
F
G
H
J
K
L
I
יו
P
F11
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Step 1: Given data
VIEW Step 2: Calculating the molarity of N2 and O2
VIEW Step 3: Calculating the Qc for the reaction
VIEW Step 4: Constructing the ICE table and writing the equilibrium constant expression
VIEW Step 5: Calculating the value of x
VIEW Step 6: Calculating the concentration at equilibrium
VIEW Solution
VIEW Trending nowThis is a popular solution!
Step by stepSolved in 7 steps with 22 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Enter your answer in the provided box. The equilibrium constant K, for the equation 2H,(g) + CO(g) =CH;OH(g) is 23 at a certain temperature. If there are 1.81 x 102 moles of H, and 2.31 x 10-3 moles of CH3OH at equilibrium in a 6.75-L flask, what is the concentration of CO? Marrow_forward4 PH3(g) 6 H,(g) + P4(g) the equilibrium concentrations were found to be [PH,] = 0.250 M, [H,] = 0.570 M, and [P] = 0.750 M. %3D What is the equilibrium constant for this reaction? Ke =arrow_forward12) If 1.34 moles each of Br2 and Cl2 are introduced into an 11.0 liter flask and allowed to come to equilibrium, what should the equilibrium concentrations of Br2, Cl2, and BrCl be? 2 BrCl (g) « Br2 (g) + Cl2 (g)Kc = 0.143arrow_forward
- Consider the following equilibrium for which H = -114.44: 4 HCl(g) + O2(g) 2 Cl2(g) + 2 H2O(g) How will each of the following changes affect an equilibrium mixture of the 4 gases in this reaction?(a) O2(g)) is added to the system. The equilibrium will not shift The equilibrium will shift toward product but Keq will not change The equilibrium will shift toward reactant but Keq will not change The equilibrium will shift toward product but Keq will will increase The equilibrium will shift toward reactant but Keq will decrease(b) The reaction mixture is cooled. (c) The volume of the reaction vessel is reduced by 50%.arrow_forwardusing the equilibrium constant expression 2H2(g)+S2(g)---->2H2S(g). Calculate the equilibrium constant if: [H2]=2.1*10-1 M [S2]=1.1*10-6 M [H2S]=7.3*10-1 Marrow_forwardPredict the equilibrium concentration of CO: in the reaction described below by constructing an equilibrium expression for Qc, constructing an ICE table, writing an equilibrium expression for Kc, and solving for the equilibrium concentration. Complete Parts 1-4 before submitting your answer. Co(g) + H:O(g) = CO:(g) + H:(g) 1 2 3 4 NEXT > In a 2.0 L container at 700 K, 0.13 mol CO, 0.56 mol H:O, 1.62 mol CO:, and 0.43 mol H: are allowed to react. Set up the expression for Qc. Each reaction participant must be represented by one tile. Do not combine terms. Once the expression is constructed, solve for Qc to determine the direction of the reaction. Qc 5 RESET [0.13] [0.56] [1.62] [0.43] [0.065] [0.28] [0.81] [0.22] [0.26] [0.13] [1.62] [0.065) [0.28] 0.10 96 3.1 9.8 ||arrow_forward
- Consider the following equilibrium: N₂O4(g) = 2NO2(g) A 1.00 L container is initially filled with 0.200 mol N₂O4At equilibrium, 0.160 mol NO₂are present. What is the equilibrium concentration of N₂O4? 0.120 mol/L 0. 040 mol/L O.0.160 mol/L 0.080 mol/L 0.100 mol/Larrow_forwardPredict the equilibrium concentration of NH in the reaction described below (for which Kc = 1.210 at the reaction temperature) by constructing an ICE table, writing the equilibrium constant expression, and solving for the equilibrium concentration. Complete Parts 1-3 before submitting your answer. = NHSH(s) NH¸(g) + H₂S(g) Using the data from your ICE Table (Part 1), construct the expression for the equilibrium constant, Kc. Each reaction participant must be represented by one tile. Do not combine terms. KC = II = 1.2 × 10-4 RESET [x] [2x] [2x]² [1.2 × 104+x] [1.2 × 104-x] [1.2 × 104 + 2x] [1.2 × 104 -2x] [1.2 × 10+ + x]² [1.2 × 10+ - x]² [1.2 × 10+ + 2x]² [1.2 × 104 -2x]²arrow_forwardPredict the equilibrium concentration of O₂ in the reaction described below by constructing an equilibrium expression for Qc, constructing an ICE table, writing an equilibrium expression for Kc, and solving for the equilibrium concentration. Complete Parts 1-4 before submitting your answer. N₂(g) + O₂(g) = 2 NO(g) 4 NEXT > In a 1.0 L container at high temperature, 0.20 mol N₂ and 0.15 mol O₂ are allowed to react. Set up the expression for Qc. Each reaction participant must be represented by one tile. Do not combine terms. Once the expression is constructed, solve for Qc to determine the direction of the reaction. [0] [0.050] 0.030 [1.0] [0.201² 1 9.0 x 10-4 Qc = [0]² [0.15]² 1.1 x 104 2 [1.01² [0.40]² [0.20] [0.30]² 3 [0.15] [0.050]² [0.40] 0 ✓ RESET [0.30] 33arrow_forward
- During an experiment, O.257 mol of H2 and 0.257 mol of I2 were placed into a 1.28 liter vessel where the reaction H2(g) + 12(g) 2 2HI(g) came to equilibrium. For this reaction, Kc = 49.5 at the temperature of the experiment. What were the equilibrium concentrations of H2, 12, and HI? [H2] = M %3D [12] = M [HI] = i Marrow_forwardMOving to anotrie question will save this response. Question 7 A state of dynamic equilibrium, Ag2CO3(s) +→ 2Ag*(aq) + CO32(aqg), exists in solutida. Which direction will the equilibrium shift if Ca(NO3)2(aq) is added to the system? O To the left. O To the right. O It will not shift left or right. A Moving to another question will save this response. MacBook Pro 80 888 FS F6 F7 FA esc F2 %23 2$ 8. 2 3 4. Q K A D 5arrow_forwardConsider the following equilibrium. N2(s) + O2(g) 2 NO(g) At 2300 K, the equilibrium constant K - 1.7 x 10. Suppose that 0.015 mol NO(g), 0.16 mol N, (9), and 0.16 mol O, (9) are placed into a 10.0 L flask and heated to 2300 K. (a) Is the system at equilibrium? If the system is not at equilibrium, in which direction must the reaction proceed to reach equilibrium? The system is at equilibrium. OThe system is not at equilibrium. The reaction must proceed to the left. The system is not at equilibrium. The reaction must proceed to the right (b) Calculate the equilibrium concentrations of all three substances. (Enter unrounded answers.) [02 - [NO Marrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY