
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please fill out all part!!! I need help!!!
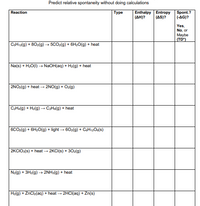
Transcribed Image Text:Predict relative spontaneity without doing calculations
Enthalpy Entropy
(AH)?
Spont.?
(-AG)?
Reaction
Туре
(AS)?
Yes,
No, or
Maybe
(TD")
CSH12(g) + 80z(g) → 5CO2(g) + 6H20(g) + heat
Na(s) + H2O(1) → NaOH(aq) + H2(g) + heat
2NO2(g) + heat – 2NO(g) + Oz(g)
C2H«(g) + Hz(g)→ C2Helg) + heat
6CO:(g) + 6H20(g) + light - 60z(g) + CEH12O6(s)
2KCIO:(s) + heat → 2KCI(s) + 302(g)
N2(g) + 3H2(g) → 2NH3(g) + heat
H2(g) + ZnClz(aq) + heat - 2HCI(aq) + Zn(s)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please correct answer and don't use hand ratingarrow_forwardInterface/acellus_engine.html?ClassID=845449353 How many moles of Al are present in 1 mole of Al₂S3 ? moles Al Ernational Academy of Science. All Rights Reserved. Ri 5 A 6 Moles Al O 2 & 7 PrtScn 8 Enter Homearrow_forward1) Using the formulas in this lab module for various shapes, calculate the volume of a solid ball made of concrete whose radius from core to outward point is 45mm. Show all work. smulov sr heqxe how lle won2 ligarrow_forward
- The difference between rubber bands and car tyres is that: a.)Tyres are made through a process of romulisation b.)Rubber bands are made through a process of romulisation c.)Tyres have more cross links than rubber bands d.)Rubber bands have more cross links than tyres doarrow_forward4- The four wires shown below are made from the same resistive material. Show the highest and lowest resistance wire. Explain your answer. A B 2L 2R C 2L 2R D ------arrow_forwardThe rotovap works to efficiently evaporate solvents by ________. reducing pressure heating spinning vibrating Reducing pressure, heating, and spinning Reducing pressure, heating, spinning, and vibratingarrow_forward
- Graphene, is a sheet of pure carbon with a thickness of 0.335nm. How many layers of graphene will be required to make 0.2 cm thick sheet? (1nm = 1x and 100cm =1m) A) 5.97 x 10^6 B) 5.97 x 10^7 C) 1.68 x10^6 D) 1.68 x10^7 E) 3.35 x10^6arrow_forwardPart A One kind of polyester is a condensation copolymer formed between terephthalic acid and ethylene glycol (Figure 1). Draw the structure of the dimer. [Hint: Water (circled) is eliminated when the bond between the monomers forms.] Draw the molecule on the canvas by choosing buttons from the Tools (for bonds and charges), Atoms, and Templates toolbar= gure 206 Terephthalic acid HO–CH,CH, OH Ethylene glycol 1 of 1 050 Δ HD EXP CONT. * Submit Previous Answers Request Answer × Incorrect; Try Again; 5 attempts remaining Part B Complete previous part(s) Provide Feedback H C S CI Br 1arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY