
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
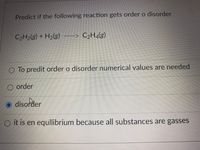
Transcribed Image Text:Predict if the following reaction gets order o disorder
C2H2(g) + H2(g)
C2H4(g)
To predit order o disorder numerical values are needed
O order
O disorder
O it is en equllibrium because all substances are gasses
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- JOP3JH-IBch9hvCIbYq_fi3Zsn8H2oW_5PT... O KINETICS AND EQUILIBRIUM Using Le Chatelier's Principle to predict the result of changing. OOO OD 35 Nerbs Methane and chlorine react to form chloroform and hydrogen chloride, like this: CH(9)+3Cl,(9) → CHCI;(g)+3HCl(g) The reaction is exothermic. Suppose a mixture of CH, Cl,, CHCI, and HCl has come to equilibrium in a closed reaction vessel. Predict what change, if any, the perturbations in the table below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the right or left, perturbation do change in composition shift in equilibrium Oto the right The temperature is lowered. The pressure of Cl, will O to the left O (none) O to the right The temperature is raised. The pressure of HC will O to the left O (none) Emanation Check 02022 Mraw H LLC. ARights Reserved Terme of Use Ivcy Center Accessbity P Type here to search 99+ IA F2 F3 F7 F8 19 F10 Fi 712 Pt Sc Insert Colete 24 4. %23 & 3 6 7 8…arrow_forwardq27) very urgnatly required pls helparrow_forwardImagine you have a water bottle that is partially filled with water and sealed. Which situation means that the system has reached equilibrium? O The rate of condensation is greater than the rate of evaporation. O The rate of evaporation is zero. O The rate of evaporation is greater than the rate of condensation. O The rate of evaporation is equal to the rate of condensation. • Previous Next>arrow_forward
- SO3 SO2 02 10 15 20 Time (min) a) Which substance(s) is/are the reactant(s)? b) Which substance(s) is/are the products(s)? c) At approximately what time does the reaction reach equilibrium (include units)? d) Write a balanced equilibrium equation that represents the graph. Be sure to take into account relative rates of disappearance/appearance. Don't forget those double-headed arrows. Concentrationarrow_forward2CO, 4 2CO + 2CO+ O2 Consider the reaction above. If pressure is increased on this equilibrium, the amount of O, wll Oremain the same O increase O decreasearrow_forwardFor the reaction H2(g) + Br2(g) =2 HBr(g), Kc = 81.4 at 385°C. If [H2] = [Br2] = [HBr] = 2.4 x 10-4 M at 385°C, which one of the following is correct? %3D [HBr] increases as the system approaches equilibrium. [H2] and [HBr] decreases as the system moves toward equilibrium. [H2] and [Br2] increases as the system approaches equilibrium. There is not enough information given to make a determination. [HBr] and [Br2] increases as the system approaches equilibrium. The system is at equilibrium.arrow_forward
- The elementary reactionearnin2H, 0(g ) = 2H2(g) + 02(8)millanproceeds at a certain temperature until the partial pressures of H, O, H2, and O, reach 0.0350 atm, 0.00600 atm, and = 0.00500 atm, respectively. What is the value of the equilibrium constant at this temperature?arrow_forwardPage < Chem 105: Chapters 17 and 16 Homework Name: Write the equilibrium expression for each of the following reactions. a. 203(g)=30₂(g) wwarrow_forwardDinitrogen tetraoxide partially decomposes according to the following equilibrium: N2Oatg) 2NO219) A 1.00L flask is charged with 0.479 moles of N204. At equilibrium at 380K, 0.176 moles of N204 remains. K. for this reaction is Write your answer to 3 Sig Figs (ie. 1.23EO)arrow_forward
- III O Kinetics and Equilibrium Writing the concentration equilibrium expression for a heterogeneous... Write the concentration equilibrium constant expression for this reaction. Cr₂0 (aq)+61 (aq)+14 H(aq)-31,(s)+2 Cr (aq)+7H₂O(1) 号 G 0/5arrow_forwardO KINETICS AND EQUILIBRIUM Dariana Using the small x approximation to solve equilibrium pro... A chemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 2 CH4(g)= 3 H,(g) + C,H2(g) K„=2. × 102 He fills a reaction vessel at this temperature with 12. atm of methane gas. Use this data to answer the questions in the table below. olo Can you predict the equilibrium pressure of H,, using only the yes 2' x10 Ar tools available to you within ALEKS? no If you said yes, then enter the equilibrium pressure of H, at right. Round your answer to 1 significant digit. || atmarrow_forward*** S 19. Solid ammonium hydrogen sulfide is introduced into a 2.00-L flask, and the flask is şealed. If this solid decomposes according to the equation below DAUS Ca Nothange NH4HS(s) NH3(g) + H₂S(g), Kp = 0.108 at 25°C, what is the minimum mass of ammonium hydrogen sulfide that must be present in the flask initially if equilibrium is to be established at 25°C? A) 0.917 g B) 1.37 g C) 2.74 g D) 0.581 g E) 0.452 garrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY