
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Draw the entire mechanism and add Curved Arrows to show clearly how electrons are
redistributed in the process.
redistributed in the process.
Please explain and provide steps clearly.
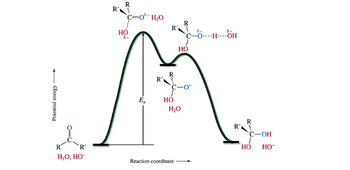
Transcribed Image Text:Potential energy —
R
H₂O,
R'R
HO
6-
C-0¯H₂O
R'
R
R
HO
C-C
8-
8-
C-OH-OH
M
HO
Ea
HO
H₂O
R'
C-OH
'R'
HO
но-
Reaction coordinate
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- 7-17 If a certain reaction takes 16 h to go to completion at 10°C, what temperature should we run it if we want it to go to completion in 1 h?arrow_forward7-46 (Chemical Connections 7D) What reaction takes place when sunlight hits the compound silver chloride?arrow_forwardDetermine rxnH 25 C for the following reaction: NO g O2 g NO2 g This reaction is a major participant in the formation of smog.arrow_forward
- complete the following reactionarrow_forward1) Н—С—-Н 2) CH2 3) 4) 5) 6) он На, Pt NANH2 HаОг, NaOH, H0 CH;Br H2, Lindlar's catalyst CrOз, H>SO4, HzO снCH,Br NaBH, NaOH CH;CH2CH;Br Вн-THFarrow_forwardSelect the reactions that can be represented with the energy diagram in Figure 12. * Reaction A Reaction B Reaction Carrow_forward
- Adentity the productis) of the following reaction. 1. KMO,/N/heal 2 HO • 2 CO COOH COOH 2 CO2 CO2 COOH OHarrow_forwardCaluclate change H for the reaction 4 NH3 (g) + 5 O2 (g) -> 4 NO (g) + 6 H2O (g).arrow_forwardTrimethylamine, (CH3)3N, is a common reagent. It interacts readily with diborane gas, B₂H6. The latter dissociates to BH3 and this forms a complex with the amine, (CH3)3N→BH3. The reaction between trimethylamine and borane is shown. CH3 CH3 H CH3-N : + B-H CH3-N B-H CH3 H CH3 H Is the BH3 fragment a Lewis acid or a Lewis base? To decide this, answer the following questions: H a Which of the following is a definition of a Lewis acid? proton donor electron pair acceptor proton acceptor electron pair donorarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning