
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
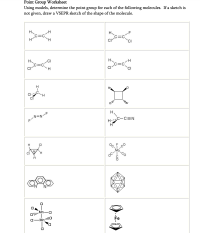
Transcribed Image Text:Point Group Worksheet
Using models, determine the point group for each of the following molecules. If a sketch is
not given, draw a VSEPR sketch of the shape of the molecule.
H
H
C=C
H.
Ha
CI
C=c
H.
Br
CI
H.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- I. Determine the point groups of the following: (found in NH, 1,) D. Tellurium tetrachloride A. Tetrafluoracubane II. Determine the following direct product representations, reducing any reducible ones. 1. On, T1u X Eg D3d, A1g X A2u X Eu 2.arrow_forwardWhat is the point group of the following molecule? A)C3v B)C2h C)C3d D)C2v E)C4varrow_forwardWhat is the point group of the following molecule? A)D3h B)C5v C)C1 D)D2h E)C2harrow_forward
- 1. Pyridine (shown below) is a planar delocalized molecule Similar to benzene H 1) Identify its principal rotation axis (draw it it on picture) 11) Determine its point group and give your resoning / explainin H H C IC H 。 H and label 1211arrow_forward3. Assign each molecule or ion to its proper point group. a. cyclobutane b. trans-1,3-difluorocyclobutane C. bromoethane d. 1,2-dibromoethane (in the anti conformation)arrow_forward1. Give the point group for each of the following molecules: H H H CI F F H H. CI BrH BrH Br Br Br Br CI H. Сyclohexane (chaй) Cyclohexane (boat) Ferrocene (staggard) Ferrocene (eclisped) 2. Give the point group for each of the following atomic and molecular orbitals: 88 dy d22 o bond n bond z antibond sp3arrow_forward
- On one-electron oxidation of a five valence electron AH₂ compound (C₂, point group) to a closed shell (no unpaired electrons) four valence electron C₂, cationic species, what is the symmetry of the highest energy occupied molecular orbital, and what happens to the energy of this orbital when the four valence electron AH2 C2y cation changes to a Doch geometry? O 2a₁ and stabilised (decreases in energy) O 2a₁ and destabilised (increases in energy) O 1b2 and stabilised (decreases in energy) O b₁ and no change in energyarrow_forward3. Assign each molecule or ion to its proper point group. a. cyclopropylene b. aziridine (hint: be very mindful of lone pairs) c. NbF,2-arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY