
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please solve question number 8 and 10.
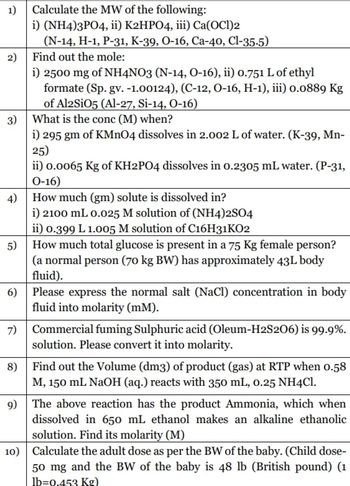
Transcribed Image Text:1)
2)
Calculate the MW of the following:
i) (NH4)3PO4, ii) K2HPO4, iii) Ca(OCI)2
(N-14, H-1, P-31, K-39, O-16, Ca-40, Cl-35-5)
Find out the mole:
i) 2500 mg of NH4NO3 (N-14, O-16), ii) 0.751 L of ethyl
formate (Sp. gv. -1.00124), (C-12, O-16, H-1), iii) 0.0889 Kg
of Al2SiO5 (Al-27, Si-14, O-16)
3) What is the conc (M) when?
i) 295 gm of KMnO4 dissolves in 2.002 L of water. (K-39, Mn-
25)
ii) 0.0065 Kg of KH2PO4 dissolves in 0.2305 mL water. (P-31,
0-16)
4) How much (gm) solute is dissolved in?
i) 2100 mL 0.025 M solution of (NH4)2SO4
ii) 0.399 L 1.005 M solution of C16H31KO2
How much total glucose is present in a 75 Kg female person?
(a normal person (70 kg BW) has approximately 43L body
fluid).
6) Please express the normal salt (NaCl) concentration in body
fluid into molarity (mM).
7)
Commercial fuming Sulphuric acid (Oleum-H2S206) is 99.9%.
solution. Please convert it into molarity.
Find out the Volume (dm3) of product (gas) at RTP when 0.58
M, 150 mL NaOH (aq.) reacts with 350 mL, 0.25 NH4Cl.
The above reaction has the product Ammonia, which when
dissolved in 650 mL ethanol makes an alkaline ethanolic
solution. Find its molarity (M)
10) Calculate the adult dose as per the BW of the baby. (Child dose-
50 mg and the BW of the baby is 48 lb (British pound) (1
lb=0.453 Kg)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 30 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY