
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please show all the calculations.
I have these information available:
32.00 mL of 1 butanol and 20.00 mL glacial acetic acid were in the 100 mL distilling flask.
Initial Aliquot:
1 mL and add around 20 mL of water.
Volume of NaoH required for titration: 67.3 mL
After Aliquot:
1 mL and add around 20 mL of water.
Volume of NaOH required for titration: 23.4 mL
Concentration of NaOH: 0.0972 M
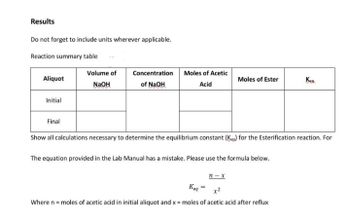
Transcribed Image Text:Results
Do not forget to include units wherever applicable.
Reaction summary table
Volume of
Aliquot
Concentration
of NaOH
Moles of Acetic
Acid
Moles of Ester
Kent
NaOH
Initial
Final
Show all calculations necessary to determine the equilibrium constant (Kg) for the Esterification reaction. For
The equation provided in the Lab Manual has a mistake. Please use the formula below.
n-x
Keq
Where n = moles of acetic acid in initial aliquot and x = moles of acetic acid after reflux
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A multivitamin sample has a label claim of 500 mg ascorbic acid (176.12 g/mol). According to quality assurance guidelines for stability, it has an acceptable range of 90.0%-110.0% of the label claim. After production, the multivitamin was analyzed through acid-base titration, and the sample needed 25.40 mL of a 0.1101 M NaOH titrant to reach the end point. After 3 months, the same multivitamin was analyzed again, and this time 23.34 mL of the same 0.1101 M titrant was used. After 6 months, the same multivitamin was analyzed again, and this time, 22.48 mL of the same 0.1101 M NaOH titrant was needed to reach the end point.arrow_forwardPreparation and Standardization of KMnO4 solution Experimental data Complete the table below. Trial 1 0.2001 g Trial 2 0.2065 g Trial 3 Weight of sodium oxalate (Na2C2O4, MM= 134 g/mol) Titration data 0.2050 g Final reading Initial reading 29.86 mL 0.00 mL 30.66 mL 30.52 mL 0.00 mL 0.00 mL Total vol. of KMNO4 used Computed Molarity of KMNO4 solution Mean Molarity Computed Normality of KMNO4 Mean Normality of KMNO4 solution Reaction Involved: Calculations:arrow_forwardI need the answer as soon as possiblearrow_forward
- Fill in the blank.arrow_forwardCalculate the mass of acetic acid in 100ml of both samples in vinegar. given: NaOH vs CH3COOH Burette solution is NaOH and the pipette solution is 5.0 mL of Vinegar Titration Initial burette reading Final burette reading Volume of NaOH consumed Average volume of NaOH Approximate 0.0 mL 20.1 mL 20.1 mL 20.3 mL Titration 1 0.0 mL 20.9 mL 20.9 mL Titration 2 0.0 mL 20.0 mL 20.0 mL To find the average volume Average volume = 20.1 mL + 20.9 mL + 20.0 mL320.1 mL + 20.9 mL + 20.0 mL3 = 20.3 mL Concentration of Vinegar = Volume of NaOH * Concentration of NaOHVolume of vinegarVolume of NaOH * Concentration of NaOHVolume of vinegar = 20.3 mL * 0.0647 M5.0 mL20.3 mL * 0.0647 M5.0 mL = 0.2627 M Concentration of NaOH = Volume of H2SO4 * Concentration of H2SO4Volume of NaOHVolume of H2SO4 * Concentration of H2SO4Volume of NaOH = 10.0 mL…arrow_forwardFor your fine titrations, after reaching the x-1 mL mark from your rough titration, you should add EDTA in 1 mL increments until you reach the endpoint. True Falsearrow_forward
- C. Titration of acid by a base Standardization of NaOH Volume of NaOH Mass of KHP Initial buret Final buret Trial used Molarity of NaOH (g) reading (mL) reading (mL) (mL) (1) (2) (3) (4) (5) 1.2075 0.7 25 24.3 0.243 (6) (7) (8) (9) (10) 1.2062 16.4 40.9 24.49 0.2412arrow_forward1. A mixture containing only KCl and NaBr is analyzed by the Mohr Method. A 0.3172-g sample is dissolved in 50 mL water and titrated to the Ag2CrO4 endpoint, requiring 36.85 mL of 0.1120 M AgNO3. A blank titration requires 0.71 mL of titrant to reach the same endpoint. Report the % (w/w) KCl and NaBr in the sample. [Ans. 84.41 % (w/w); 17.59 % (w/w)]arrow_forwardPost Lab Assignment 1. Titrate 15.00 mL of 0.256 M KHP solution requires 20.75 mL of an unknown NaOH solution to reach the equivalence point. What's the molarity of this unknown NaOH solution? Show your work. 2. Titrate 1.2035g of solid KHP (KC,H,04) requires 23.89 mL of an unknown NaOH solution to reach the equivalence point. What's the molarity of this unknown NaOH solution? Show your work.arrow_forward
- In Dissolution of Borax experiment, the standard HCI solution has a concentration of 0.130 mol/L. The initial buret reading is 4.24, and the final buret reading is 33.38. The volume of the saturated borax solution is 5.34 mL. What is the molar solubility of borax? Note: Report both number and unit. Pay attention to significant figures.arrow_forwardWhat is Analyte and Titrant? Explain each in 3-5 sentences.arrow_forwardPlease send me the question in 30 minutes it's very urgent plzarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY