
Chemistry: Principles and Practice
3rd Edition
ISBN: 9780534420123
Author: Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please answer with All the required steps needed to solve it
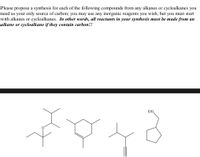
Transcribed Image Text:Please propose a synthesis for each of the following compounds from any alkanes or cycloalkanes you
need as your only source of carbon; you may use any inorganic reagents you wish, but you must start
with alkanes or cycloalkanes. In other words, all reactants in your synthesis must be made from an
alkane or cycloalkane if they contain carbon!!
EtO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please help me check if the information below is correct for both the types of reactions and the special rules or laws to predict predominant products for alcohols. If not please insert the correct information. Please make the information in jot notes. TYPES OF REACTIONS ALCOHOL: Dehydration: This is a reaction where an alcohol loses a water molecule to form an alkene. For example, when ethanol is treated with an acidic catalyst, such as sulfuric acid, it undergoes dehydration to form ethene (CH2=CH2) and water. Oxidation: In this reaction, an alcohol is converted to either a carbonyl compound or a carboxylic acid. For example, primary alcohols can be oxidized to aldehydes or carboxylic acids, while secondary alcohols can be oxidized to ketones. Tertiary alcohols are not oxidized under normal conditions. Esterification: This reaction involves the formation of an ester from an alcohol and a carboxylic acid in the presence of an acid catalyst. For example, when ethanol is…arrow_forwardPlease be clear in your writing The following names may have some errors. Correct the name and render the structures corresponding to the following names. g) 1,3-pentadiino h) cyclohexylacetylenearrow_forward5B In the following reactions, mixtures of alkenes and ethyl ethers are formed. Draw their structures. Explain which is or are likely to be the main product(s) in each reaction. In case of formation of two isomers of alkenes, explain which is formed in greater proportion CH3 CH3 H3C-C H -Br CH3 EtOHarrow_forward
- Hello, The answer to the second question was incorrect. "You have incorrectly provided the IUPAC name. For the common name of disubstituted benzene compounds, the relative position of the substituents must be defined using the terminology ortho-, meta-, para-." Could you please try again? Thank you!arrow_forwardThe acid-catalyzed dehydration of 2,3-dimethyl-3-pentanol yields three alkene products. What are the names of the three alkenes? Which of the three alkenes is the major product?arrow_forwardCCH H20, H2SO4 H9SO4 CH3 Alkynes do not react directly with aqueous acid as do alkenes, but will do so in the presence of mercury(II) sulfate as a Lewis acid catalyst. The reaction occurs with Markovnikov regiochemistry, so the OH group adds to the more highly substituted carbon and the H adds to the less highly substituted carbon. The initial product of the reaction is a vinyl alcohol, also called an enol. The enol immediately rearranges to a more stable ketone via tautomerization. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions Hjö: -CH3 -CH3 H3O*arrow_forward
- Which alkane most readily undergoes thermal decomposition? Note that C-H bonds are usually stronger than C-C bonds. O ethane O dimethylpropane O propane O methylpropanearrow_forwardDraw a structural formula for the product formed upon hydroboration/oxidation of the alkene below.arrow_forwardPl V In consideration of the following organic compound names and/or descriptors, which one is the correct IUPAC name for the compound structure given at right? If none of these is the correct IUPAC name for this structure, select NONE. OA 2-methyl-3-butyne OB. 3,3-dimethylpropyne OC 3-methyl-1-butyne OD. NONE «< Question 1 8 U K B. Marrow_forward
- PARTIAL OR eomPLETE ComB USTION O N-HEXANE O CYCLO HEXENE PHENOL O GASOLINE VATURATOD OR UNSATURATED O N-HEXANE CYCLO HEXENE PHENOL O LASOUNE ALIPHATIC OR AROMATIC HYPROCARBONS O N-HBXANB D CYCLOHEXENG O PHBNOL 12 GASOUNB FOUR MAJOR CLASSES OF HNOROCARBONS ( ALKANES, ALKENBS, ALKYNBS, AROMATIC) O N-HBXANE D CYCLOHEXBNG D PHBN DL 10 GASOUNEarrow_forwardKinetics and thermodynamics H+ catatylzes the esterification reaction of alcohols with carboxylic acids. Describe at least two ways the addition of H+ can increase the rate of the reaction. based on the imagearrow_forwardA hydrocarbon of unknown structure with the formula CgH14 was isolated. In an effort to determine the structure, the compound was subjected to hydrogenation with H2 and Pd, which afforded a product with a formula of C8H16 Which of the following compounds are possible candidates for the starting hydrocarbon?@GMU 2020 Me II II Mearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
