
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
7
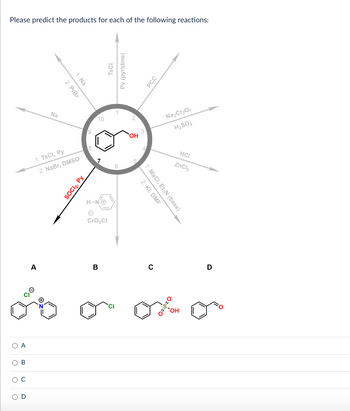
Transcribed Image Text:Please predict the products for each of the following reactions:
O A
O
C
O
B
D
Na
A
2. PrBr
1. Na
1. TsCI, Py
2. NaBr, DMSO
SOCI₂, Py
9
10
H-NO
e
CrO₂Cl
B
TSCI
Py (pyridine)
1
2
OH
3
2. KI, DMF
1. MSCI, Et3N (base)
C
PCC
Na₂Cr₂O7
H₂SO4
HCI
ZnCl₂
OOH
D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Taking into account that the spheres represent atoms, indicate:a) if the represented systems correspond to mixtures or notb) if the substances are simple or compound.c) In what state of aggregation are they?arrow_forward13. Soru Complete the given reaction. Fe(s) + H2SO4(aq) + ........ ........ A) Fe (aq) + SO2(g) B) FeSO4(s) + H2(g) C) FeH2(aq) + H2(g) D) FeH2(aq) + SO2(g)arrow_forwardmistry F20 SE 1. If you have 23.2 grams of gold, what is the volume of the sample? Densities of Some Common Metals Metal Density (g/mL) Copper 8.94 the Aluminum 2.70 Gold 19.3 (A) 0.116 ml (B) 0.832 mL (C) 1.20 ml (D) 8.59 mL DELLarrow_forward
- what is an intensive observation of gold?arrow_forward4. (Also can you explain what is meant by determinant error)arrow_forwardNa (g) + CI(g) + e 4502 kl/mol 355 kl/mol Na(g) + CI(g) Na*(g) + CH(g) +121 kl/mol Na(g) + 1/2 Cl;(g) ki/mol -786 kl/mol Na(s) + 1/2 Cl2(g) -411 kl/mol NaCI(s) The ionization energy of Na (g) is -411 kJ/mole 502 kJ/mole -355 kJ/mole -786 kJ/molearrow_forward
- 14 14 7.6C7N+ 1H le O 00 -le OIN 14 Traveling Throuarrow_forwardSr rontium 37.62 56 Ba Barium 137.3 9 7 Fr ancium 223) Yttrium 88. F 88 Ra Radiu 22f a. 1 b. 3 c. 5.0 mL d. 2.3 mL e. 5.6 mL c. 5 9. How many milliliters of 10.0 M H₂SO4 are needed to prepare 500.0 mL of 0.10 M H₂SO4? 50 mL a. b. 78 mL d. 7 seedarrow_forwardVanadium is used in the manufacture of rust - resistant vanadium steel. It forms bcc crystals with a density of 6.11 g/cm3 at 18.7\deg C. What is the approximate metallic radius of the vanadium in picometers? Round to a whole number.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY