
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
pp
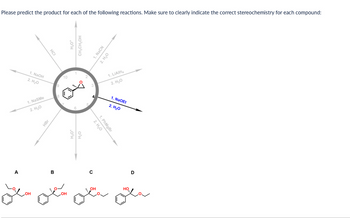
Transcribed Image Text:Please predict the product for each of the following reactions. Make sure to clearly indicate the correct stereochemistry for each compound:
A
1. NaOH
2. H₂O
1. NaSMe
2. H₂O
HCI
HBr
B
10
otá
H₂O*
CH₂CH₂OH
H₂O*
4 NaCN
2. H₂O
2. H₂O
1. PhMgBr
1. LIAIH4
2. H₂O
1. NaOEt
2. H₂O
شعل
D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In the opening scene of the movie Raiders of the Lost Ark, Indiana Jones tries to remove a gold idol from a booby-trapped pedestal. He replaces a gold (D = 19.32 g/cm²³) idol of dimensions 5.0 inches by 5.0 inches by 5.0 inches with a bag of sand of approximately double the volume with sand (D = 3.65 g/cm³). Is the bag of sand really equal to the gold idol or will Dr. Jones set off the trap? Explain your answer and include a mathematical explanation. ML Cm3arrow_forward12) Which of the following represents a chemical property of copper metal? Sobibor (11)isdom 16: Copper metal conducts heat. 19 2 Copper metal reacts with nitric acid to produce copper(II) nitrate. BELO O 20.0 Copper metal melts at 1085 °C. Copper metal conducts electricity. e enthalpy 2arrow_forwardThe mole is a unit for counting. O False O Truearrow_forward
- Chalcopyrite is among the most common varieties of copper ore. Assuming chalcopyrite is 34.4% copper by mass (34.4 grams copper per 100. grams of ore), how many kilograms of chalcopyrite would be needed to smelt 30.0 kilograms of pure copper?arrow_forwardIn 1986 an electrical power plant in Taylorsville,Georgia, burned 8,376,726 tons of coal, a national record at the time.arrow_forwardA chardonnay wine is 13.5 percent by mass alcohol. If we consume 24 fluid oz of wine, and the density of the wine is 0.982 mg/mL, how much alcohol was consumed? 09.6 x 103 g alcohol O 301 g alcohol O 94 g alcohol O 106 g alcohol O 13.3 g alcoholarrow_forward
- A sunscreen preparation contains 2.50% by mass benzyl salicylate. If a tube contains 4.0 ounces of sunscreen, determine how many kilograms of benzyl salicylate are needed to manufacture 325 tubes of sunscreen. Conversion: 2.205 lb= 1 kg. 16.0 oz = 1 lbarrow_forwardAqueous hydrobromic acid HBr will react with solid sodium hydroxide NaOH to produce aqueous sodium bromide NaBr and liquid water H2O . Suppose 34.8 g of hydrobromic acid is mixed with 13. g of sodium hydroxide. Calculate the minimum mass of hydrobromic acid that could be left over by the chemical reaction. Be sure your answer has the correct number of significant digits.arrow_forwardAsking for Q 9arrow_forward
- A chemist measures the amount of oxygen gas produced during an experiment. She finds that 95.5 g of oxygen gas is produced. Calculate the number of moles of oxygen gas produced. Round your answer to 3 significant digits. mol x10 X Śarrow_forwardHow many grams F in 511.9 g Br2F6? Put the answer in the box in regular (non-scientific) notation. Use one figure past the decimal.arrow_forwardAqueous hydrobromic acid (HBr) will react with solid sodium hydroxide (NaOH) to produce aqueous sodium bromide (NaBr) and liquid water (H2O). Suppose 73.6 g of hydrobromic acid is mixed with 19. g of sodium hydroxide. Calculate the minimum mass of hydrobromic acid that could be left over by the chemical reaction. Be sure your answer has the correct number of significant digits. g ☐ x10 ☑arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY