
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Show work. Don't use Ai for answering this
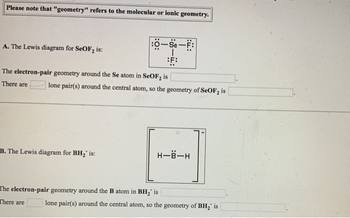
Transcribed Image Text:Please note that "geometry" refers to the molecular or ionic geometry.
A. The Lewis diagram for SeOF2 is:
:O-Se-F:
F:
The electron-pair geometry around the Se atom in SeOF₂ is
There are
lone pair(s) around the central atom, so the geometry of SeOF2 is
B. The Lewis diagram for BH2" is:
The electron-pair geometry around the B atom in BH₂ is
There are
H-B-H
lone pair(s) around the central atom, so the geometry of BH₂ is
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- please show work and circle answer for each part . Thanksarrow_forwardConsider a barrel containing one copy of each of the 26 letters of the alphabet (A, B, C, …).A. What is the probability of drawing the first three letters of the alphabet (A, B and C) out inexactly alphabetical-order if you replace each letter back in the barrel after you look at it?B. What is the probability of drawing all 26 letters of the alphabet out in exactly alphabeticalorder if you replace each letter back in the barrel after you look at it?C. What is the probability of drawing the first three letters of the alphabet (A, B and C) out inexactly alphabetical-order if you keep the letter out of the barrel after you look at it?arrow_forwardSafari File Edit View History Bookmarks Window Help () 48% Sun 10:56 AM A session.masteringchemistry.com Consider summer class... Inbox (13) - thesym1@g... Class Schedule Listing Schedule 111.009 & 111.. Ellucian Degree Works... Pearson's MyLab & Mas... ALEKS - Sofia Simmons... MasteringChemistry: H-arrow_forward
- Simplify by reducing to the fewest number of termsa(b/a)arrow_forwardSafari File Edit View History Bookmarks Window Help 50% Sun 10:49 AM A session.masteringchemistry.com Consider summer class... Inbox (13) - thesym1@g... Class Schedule Listing Schedule 111.009 & 111... Ellucian Degree Works... Pearson's MyLab & Mas... ALEKS - Sofia Simmons... MasteringChemistry: H... If a solute produces ions when dissolved, it is called an electrolyte because the resulting ions in solution will allow the solution to conduct electricity. A solute that completely dissociates into ions is a stronger electrolyte than one that only partially dissociates into ions. If a solute remains as a molecule when dissolved, it is called a nonelectrolyte.In this tutorial, you will practice identifying substances as strong electrolytes, weak electrolytes, or nonelectrolytes. Part A Each of the following reactions shows a solute dissolved in water. Classify each solute as a strong electrolyte, a weak electrolyte, or a nonelectrolyte. 1. C(1)→C(aq) 2. AB(aq) = A+(aq)+B¯(aq) 3. MN(aq)→M*…arrow_forwardA student used a pH meter to collect data for the titration of an unknown concentration of propanoic acid with a 0.150 mol/L solution of sodium hydroxide. The data table from the investigation is shown in the table below. Data for the Titration of Propanoic Acid with Sodium Hydroxide Volume of propanoic acid: 25.00 mL [NaOH] = 0.150 mol/L Volume of NaOH (aq) added (mL) 0.00 2.00 4.11 7.98 11.95 14.08 16.05 17.00 17.21 17.39 17.62 17.99 18.18 18.39 18.80 20.00 22.03 PH 2.83 3.84 4.20 4.64 5.03 5.27 5.61 5.90 6.00 6.09 6.23 6.74 8.80 10.92 12.24 12.56 12.69 Adobe 4343625416 00..arrow_forward
- If the hydrogens labeled 'a' integrate for ¹H, what integration is found for the hydrogens labeled 'b'? O O 1H 2H 3H 4H 5H ng to another question will save this response. a barrow_forwardtion 2 3 5 8 9 10 11 12 13 14 15 16 17 18 19 20 The end point and the equivalence point are always equal. O True O False 事 4.arrow_forwardWhat is the protien gradient?arrow_forward
- please give calculation as well. Calculate the pH value of each of the solutions in tubes 1-9 using the Henderson-Hasselbalch equation (H-H eqn).Determine the pl of casein. Compare your experimental values with those found in the literature. Biomolecules. Thank you! The protein is casein.arrow_forwarddemostrate curved arrows for this mechanismarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY