
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please give IUPAC systematic names for the molecules including R/S and E/Z designations
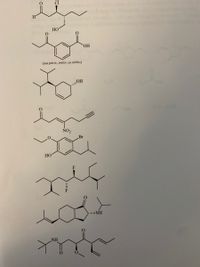
Transcribed Image Text:The image contains a series of chemical structures. Here’s a description of each:
1. **1st Structure**: A molecule with a chloro group (Cl) and a ketone (C=O) adjacent to the chloro group. There's an alcohol group (OH) nearby.
2. **2nd Structure**: A phenolic compound with a benzene ring featuring an alcohol group (OH) and two ketone groups (C=O) on either side of the ring. A note specifies the position of the groups: "use para-, meta-, or ortho-."
3. **3rd Structure**: A bicyclic compound with a hydroxyl group (OH) attached to one of the rings.
4. **4th Structure**: Contains an aromatic ring with a nitro group (NO2) and a ketone (C=O). The structure also includes a bromine atom (Br).
5. **5th Structure**: A fluorine (F) atom is attached along with complex branching, indicating substituents on a cyclohexane ring.
6. **6th Structure**: Features a bicyclic compound with a nitrogen-containing group. The structure includes a methyl ketone and an amide group (NH).
7. **7th Structure**: A compound with several carbon and oxygen-containing functional groups, featuring an amide group (NH2) and multiple branched alkyl groups.
These structures represent various organic molecules, illustrating different functional groups and steric configurations useful for educational purposes in chemistry.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the following reaction. Part: 0 / 2 Part 1 of 2 Cl₂ hv 16-0 Draw the skeletal structure of all organic products (including stereochemistry) of the monochlorination reaction. Consider attack at C1 and C3 only. Hydrogens on chiral carbons should not be included.arrow_forward↑ ↓ Inbox - ss997 app.101edu.co Orgo H₂O Orgo - Googl X Drawing will readily Unexpectedly, 7-bromocyclohepta-1,3,5-triene dissolve in water. Draw the aromatic structure that forms in aqueous solution. Include all lone pairs in your structure. Br: Window B Grades - Org x Q Help Benzerarrow_forward10arrow_forward
- place the following alkanes in order of increasing boiling point. PLEASE write it down A, B or Carrow_forward#11arrow_forwardA compound was found to have the molecular formula C5H12O and could be any of the compounds shown below. To determine the identity of the unknown, a series of chemical tests were conducted. Assign each test result to the correct compound. Further, the oxidation product of the unknown was able to turn blue litmus paper into red. Determine its identity. Type in the blanks the set of CAPITAL LETTERS corresponding to your answer. OH Į Test MSH reagent/result CrO3, H₂SO4 Conc. HCI, ZnCl₂ Aq. FeCl3 OH Reaction with Na metal (evolution of H₂) Cpd (1): SHM Cpd (2): + ++ ++ IDENTITY OF THE UNKNOWN: OH Xx MHS Cpd (3): + HMS Cpd (4): +++ +++arrow_forward
- Chemistry F₁₁, CH3 Br FF CH3 Do I of CH₂CI CH3 H₂N H H3C CH3 OH Determine the absolute configuration (R/S) of the stereogenic carbon of the following compoundsarrow_forwardPlease provide the reason why the chosen answer is correct! HOW MANY different types of DIETHYLBENZENE are there, and what are their names? three: 1,3-diethylbenzene, ortho-diethylbenzene, m-diethylbenzene one: diethylbenzene three: meta-diethylbenzene, 1,2-diethylbenzene, p-diethylbenzene four: 1,1-diethylbenzene, m-diethylbenzene, 1,4-diethylbenzene, ortho-diethylbenzene two: cis-diethylbenzene, trans-diethylbenzenearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY