
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Please explain the relationship between the Air-Fuel ratio and the emission of CO, HC, And NOx From Automobiles, as shown in the graph.
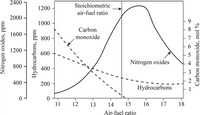
Transcribed Image Text:2400
Stoichiometric
1200
air-fuel ratio
2000
1000
9.
Carbon
1600 -
800
monoxide
1200
600
Nitrogen oxides
4
800
400
400
200
Hydrocarbons
11
12
13
14
15
16
17
18
Air-fuel ratio
Nitrogen oxides, ppm
Hydrocarbons, ppm
Carbon monoxide, mol %
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- 2. You are working for a power plant that makes use of coal tar, a type of liquid fuel as its raw material for burning in a boller. This type of fuel is mainly composed of carbolic oll, naphthalene oil, creosote oil and anthracene oll. After analyzing coal tar you were able to wow it contalns 85.9% 0,63% H, 2.2% S. 4.5% O and 1.1% N. You told the operators to make use of excess air for combustion. The air was assumed to enter at 30°C with 90%RH. In order to follow DENR regulations, you took a sample of the bumper gis to analyze its contents. Midway your experimentation, the equipment you used broke so it only gave you a partial analysis of 10.64% CO, 3.19%, CO and 0.64% H. a. The operators had no idea how much excess alr was needed, What do you tell them? b. You need to make the report to be passed that day for the analysis of the burner gas. What analysis will you submit the volume of the bumer gas in m/ka tuel is aso needed in your report c. The alr to be supplied a controlled…arrow_forward4. Pure octane has a boiling point of 125.7 °C, but can be steam distilled with water at a tem- perature of 90 °C. Calculate the mass of octane that codistills with each gram of water and the percent composition of the vapor that is produced during the steam distillation. [Again, you'll need the vapor pressure of water at the steam distillation temp to solve this problem!] Note that if you multiply the second equation from question 3 above by the MW of both the oil and water, you get: mass oil/mass water = (P°) (MW)/ (Pwater) (MW water)arrow_forwardUsing RCRA procedures, determine if the following are hazardous wastes. State the reason why, or why not it is hazardous. If it is hazardous, state the category number. Assume the industry producing the waste is a RCRA hazardous waste generator. 1. A drum of sulfuric acid at pH 1.2. A drum of waste trichloroethylene (22% by volume).arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The