
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please explain the number of signals and the integration that is observed in the 1H NMR spectrum for the major product of the reaction from question 6. - Focus on the alkyl protons, in particular those two methylene (CH2) groups
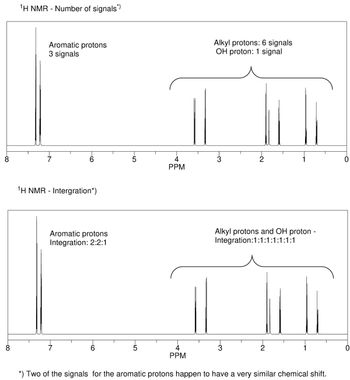
Transcribed Image Text:8
8
¹H NMR - Number of signals*)
Aromatic protons
3 signals
7
O)
¹H NMR - Intergration*)
Aromatic protons
Integration: 2:2:1
7
-CO
6
·LO
5
-LO
5
4
PPM
4
PPM
Alkyl protons: 6 signals
OH proton: 1 signal
3
2
3
Alkyl protons and OH proton -
Integration:1:1:1:1:1:1:1
-~
1
2
1
*) Two of the signals for the aromatic protons happen to have a very similar chemical shift.
0
0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the mechanism of the reaction for 4 - bromoanilinium chloride with sodium hydroxide. Make sure all species are balanced. (hint: it is an acid-base reaction) (PS - upload the images with the drawn mechanism for all the reactants, products and intermediates. be detailed as much as possible.)arrow_forwardUtilizing the cehmcial reaction: Write a “curved arrow” mechanism for the synthesis of the methyl methoxycinnamate prepared in this experiment.arrow_forwardWrite structures for the carbonyl electrophile and enolate nucleophile that react to give the enone below. مثله You do not have to consider stereochemistry. . Draw the enolate nucleophile in its carbanion form. Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate structures with + signs from the drop-down menu. سكر ٥٠ n ChemDoodle 4.arrow_forward
- Provide mechanism with curved arrows for Part C: Synthesis of tetraphenylcyclopentadienonearrow_forwardMany biochemical reactions utilize cationic imines (a Schiff base) as electron sinks. Illustrate your understanding of this concept by drawing the mechanism of basic imine formation between an aldehyde (RCHO) and an amine (RNH2). For full credit, you must show all electron flow (using the arrow convention).arrow_forwardA chemist performed a reaction of 2-methylpent-2-ene with HBr in diethyl ether. However, after completing the reaction, she became concerned about the ether she had used as a solvent. Sometimes ethers can contain peroxides which form radicals. Unfortunately, radicals can impact the product of the reaction. Use the 'H NMR data to determine the result of this reaction. HBr diethyl ether &(open) muldplicity nogalion 1 musplen IH 24 244arrow_forward
- What spectral features would you expect to see in MS and IR to determine whether the synthesis was fully successful/complete?arrow_forwardI need help predicting the Ir anaylsis of the product and to gove frequinces that i expect to see to predict ir absorption bands for functional groups present within the moleculearrow_forwardShow mechanistically how a hydrogen atom transfer occurs from benzaldehyde to a radical hydroxyl group (ie O radical).arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY