
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
| Unrounded | ||
|---|---|---|
| Average M HCl | 0.421203 |
| Average M NaOH | 0.090023 |
|---|
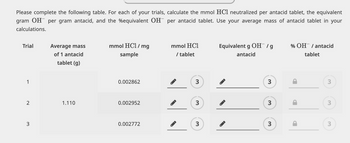
Transcribed Image Text:Please complete the following table. For each of your trials, calculate the mmol HCl neutralized per antacid tablet, the equivalent
gram OH per gram antacid, and the equivalent OH per antacid tablet. Use your average mass of antacid tablet in your
calculations.
Trial
1
3
Average mass
of 1 antacid
tablet (g)
1.110
mmol HCl/mg
sample
0.002862
0.002952
0.002772
mmol HC1
/ tablet
3
3
3
Equivalent g OH¯ / g
antacid
t
3
3
3
% OH / antacid
tablet
3
3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 9 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete this table to show the pH and pOH of each solution. Solution A Solution B Solution C Solution D Solution A [OH-] = Solution B [H+] = Solution C [OH-] = Solution C pOH = 1.0 x 10-6 1.0 × 10-11 [OH-] 1.0 × 10-10 M pH M 6 9 Solution A pOH = M Solution B pH= Solution C pH = Solution D [H+] = POH 10 17 Marrow_forwardConsider the following balanced chemical equation: Mg(OH)2 + 2HCl → 2H2O + MgCl2 How many grams of HCl would be required to neutralize 50.0 grams of Mg(OH)2?arrow_forwardHow to calculate the concentration of carbonate anion (CaCO3) in moles per liter if the concentration is 42.54 g/Larrow_forward
- What is the percentage of HCl in the solution if the molarity is 0.268398000? Assume the density of the solution is 1.00 g/mL.arrow_forwardWhat volume in liters of 0.500 M H2SO4 is required to neutralize a solution prepared by dissolving 28.5 g of KOH in 250 mL of water? Group of answer choices 0.508 1.02 0.254 0.127arrow_forward1. KMnO,/NaOH/H₂O (x) 2. H₂O* IV OHarrow_forward
- 43.27 mL of 0.5033 M NaOH are required to neutralize 29.16 mL of a H2So4 solution. What is the molarity of the H2SO4 solution? H2SO4 + 2 NaOH-> Na2SO4 + 2H2Oarrow_forwardIf 25.0 mL of a 6.00 M HCL solution is diluted to 2.00 L, what is the molarity of the new solution? Determine the volume (in mL) of water that needs to be added to 25.0 mL of a 0.250 M NaBr to produce a 0.0275 M solution. Assume the volumes are additive.arrow_forwardWhich of these is an aqueous solution? milk your exhaled breath a piece of wood 14 karat goldarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY