
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
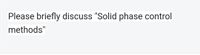
Transcribed Image Text:Please briefly discuss "Solid phase control
methods"
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Polycarbonate, PC, is used for aircraft canopies. A batch of this polymer was found to fail prematurely when impacted by a 4 pound bird during qualification studies. You suspect that the problem is due to a molecular weight difference for this particular batch of polymer compared to previous batches. You decide to perform dilute solution viscosity studies to determine the Mol.wt. Assume density is 1.2g/cm3 What solvent would you use? Justify your answer Please answer very soon will give rating surelyarrow_forwardFf.325.arrow_forwardThe viscosity of Ethyl acetate determined at 25°C was 4.2cp. The efflux time for water to flow from marks A to B was 3 minutes.Additional data: Viscosity of water at 25°C = 1.002cp Density of water at 25°C = 0.997g/mL Calculate for the time for a 10.0mL Ethyl acetate (equivalent to 5.0g) to flow from Marks A to B.arrow_forward
- help please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forward1. calculate the density of water in g/ml. 2. calculate the viscosity of water in cP. *arrow_forwardShort-circuit diffusion means that atomic migration occurs along dislocations, grain boundaries, and external surfaces. dislocations, vacancies, and external surfaces. vacancies, grain boundaries, and external surfaces. dislocations, grain boundaries, and vacancies.arrow_forward
- Check this and please answer it as soon as you can. It's very important. If you do help me, i will defitinely consider giving thumbs up https://drive.google.com/file/d/1mN-k-am1ILxrEOzVQ-8kLtrv5vK8HFvw/view?usp=sharingarrow_forwarddata collected Table 1 BINARY LIQUID-VAPOUR acetone chloform PHASE DIACRAM Temperature °c mol fraction Acetone pure mol fraction 50°℃ 1,3593 IL 1,3579 RICH-SIDE 53,5°c 2V 1,3646 2L 1,3676 RESUME 56,5°℃ 3V 1,3659 3L 1,3705 ADA CHLOROForm 57,5°C BIV 3738 4L 1,3819 ADD CHLOROFORM 58,5 SV 1,3841 SL 1,3958 AZEOTROPE 76 GV 1,3900 6L 1,4087 CHLOROFORM PURE 62 Tov 1,4464 7L 1,4518 RICH-SIDE 58,5 V 1,4352 8L 1,4336 60 AZEOTROPE 62 10 QV 1,4166 9L 1,4143 10L 1,4164 1,4166 2. 3. Plot the temperature values versus mole fraction. Draw a smooth curve through the distillate points V and another through the residue points L. Label the areas of the diagram to indicate what phases are present. Report the azeotropic composition and temperature.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY