
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
1. How many grams of Mg3(PO4)2 are in 10.00 mL of a 2.500 M solution ? (answer = 6.572 g)
Please break down and explain the question in the same following format:
Thank you!
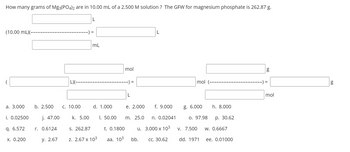
Transcribed Image Text:How many grams of Mg3(PO4)2 are in 10.00 mL of a 2.500 M solution? The GFW for magnesium phosphate is 262.87 g.
(10.00 mL)(-
a. 3.000
i. 0.02500
q. 6.572
x. 0.200
b. 2.500
j. 47.00
r. 0.6124
y. 2.67
L)(---
C. 10.00
k. 5.00
L
=
mL
d. 1.000
S. 262.87
Z. 2.67 x 10³
I. 50.00
mol
--) =
t. 0.1800
aa. 103
L
e. 2.000 f. 9.000
n. 0.02041
3.000 x 10³
CC. 30.62
m. 25.0
u.
L
bb.
mol (-
g. 6.000
0. 97.98
V. 7.500
dd. 1971
h. 8.000
p. 30.62
W. 0.6667
ee. 0.01000
g
) =
mol
g
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What mass of Fe(NO3)3 do you need to prepare 250.00 mL of .750 M solution?arrow_forward7. During week l's lab we investigated the effect of different soaking solutions on the behavior of red blood cells. If the blood had arrived on time, our prepper would have needed to know how to make up different concentrations of saline (NaCl) solution from a 2.7% stock solution of NaCl. Can you help her by filling in the table below? For each group of students, you want to make 9ml of either 0.9% saline or 0.45% saline. Show your working out below the table you complete. 0.9 0.45 % saline solution wt/vol Volume of 2.7 2.7%wt/vol NaCl in ml Volume of distilled water in mlarrow_forwardMy teacher says the answer is 0.84 M, but how?arrow_forward
- The solubility of magnesium flourish in water is 1.5*10-2 g/L. What is the solubility in grams per liter of magnesium fluoride in 0.35 M of sodium fluoride?arrow_forwardTable 4. Concentration calculations Mass of empty evaporating dish (g) 29.258 Volume of NaCl solution (mL) 10.0 Mass of dish and NaCl solution (g) Mass of dish and dry NaCl (g) Mass of NaCl solution (g) Mass of dry NaCl (g) Mass/mass percent (%) Mass/volume percent (%) Moles of NaCl (mol) Volume of NaCl solution in liters (L) Molarity of NaCl solution (mol/L) 40.498 31.164arrow_forwardIf you wanted to create a 2 mM solution of sodium chloride in 1000 mL from a 200 mM sodium chloride stock solution, what volume of the solution would you use? (hint: M1V1 = M2V2).arrow_forward
- In the lab, you used 2.0 mM FD&C Red Dye No. 40 solution. How much solid dye, molar mass 496.42 g/mol, should be dissolved to make 6.30 liters of 6.39 mM FD&C Red Dye No. 40 solution? Hint, 2.0 mM is 0.0020 M. 81.09 g 0.05004 g 0.8477 g 19.98 g 40.26 garrow_forwardHow many milliliters of 3.0 M Na3(PO4)2 are needed to react with 38.0 mL of 2.5 M MgCl2?arrow_forwardChrome File Edit View History Bookmarks People Tab Window Help * 46% (4) Tue 9:17 AM so Chem101 G how many liters in a milliliter - X + i app.101edu.co Apps M Gmail YouTube ? O Maps A Translate https://www.carth. O Google Chrome isn't your default browser Set as default Question 8 of 46 Submit How many mL of 0.100 M NaCl would be required to make a 0.0350 M solution of NaCl when diluted to 150.0 mL with water? mL 1 4 C 7 8 +/- х 100 + W dtv OCT 27 80 888 FA F3 esc & %23 %24 8. 9 5 6 7 2 3 4 P Q W E K F A > 3.arrow_forward
- Need help with this question. Thank you :)arrow_forwardDo not use chatgpt.arrow_forwardDiscussion This virtual experiment involves the determination of the conc¬entration of salt (sodium chloride, NaCl) in an aqueous solution. Concentration may be expressed many ways, and this experiment studies two systems: molarity (M) and weight/volume percent (%w/v). 1. Molarity is defined as the number of moles of solute (in this case NaCl) in a liter of solution. It is calculated with the following equation: M = moles solute liters solution 2. Percent concentration (w/v) is defined as the number of grams of solute (in this case NaCl) in 100. mL of solution. It is calculated with the following equation: %(w/v) = grams solute x 100mL solution The experimental method involves taking a specific volume of an aqueous salt solution and slowly evaporating it to dryness. The weight which remains is that of the solute. From the weight of solute and the initial volume of solution, both molarity and percent (w/v) can be calculated. Experimental In this virtual experiment, three students (A, B…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY