
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please answer this NEATLY, COMPLETELY, and CORRECTLY for an UPVOTE.
Which of the compounds given below are the most probable identities of spots A, C, F, and J?
Explain your assignment of the identities of the spots. (Probably in terms if polarity of the compounds, Rf values, etc. anything that falls under paper chromatography).
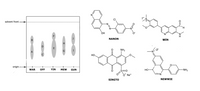
Transcribed Image Text:శింా
solvent front
OH
F
B
NANON
WIN
D
E
A
G
I
NH2
HO.
origin.
.......
WAR
OFF
YIN
MEW
GUN
но-
-NH2
0=
O Na+
SINGTO
NEWWIE
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give at least two reasons for using very dilute solutions when doing fluorescence spectrometryarrow_forward6arrow_forwardAt what m/z are peaks expected to appear for the following?1) hexane2) ethyl methyl ether3) 4-heptanone. Show the fragments detected by the spectrometer using different fragmentation patterns.arrow_forward
- 6. Which molecule will have a larger R, on a SiO₂ TLC plate using 10% Ethyl acetate/hexane as eluent? Draw their structures and explain why. Benzoic acid or ethyl benzene?arrow_forward1arrow_forwardQ.1. Predict the relative intensity of the M, M+1, and M+2 peaks for a low resolution mass spectrum of a compound with molecular formula, C6H13Br. The natural abundances of the select isotopes are given below. 35 Cl: 75.8% 79Br: 50.5% 12C: 98.89% 37Cl: 24.2% (The ratio is almost 3:1) 81Br: 49.5% (The ratio is almost 1:1) 13C: 1.11%arrow_forward
- Please Answer Part "B". Part "A" is attached for reference.arrow_forward5. Properties and IMF: Chlorophyll a and Chlorophyll b are almost identical in structure, but they separated during TLC. Report the RFs you recorded for both molecules. What functional group(s) are different between them? Then explain these RF values by comparing the IMF present in each molecule to those present in the mobile or stationary phase and how this shaped their affinities for each phase. Use the terms RF, IMF, polar, nonpolar, affinity, mobile phase and stationary phase.arrow_forwardPlease don't provide handwriting solutionarrow_forward
- Please help answering all parts of the following question...thank you! From the following sets of data, please propose a reasonable structure for the unknown organic compound. This molecule is entirely composed of C, H, and N only. Please show all of your work and feel free to write on the spectra any clues as to the structure.arrow_forwardIn the TLC experiment you are give a mixture of benzoic acid C6H5COOH and 2-hydroxy benzoic acid C6H4 ( OH)COOH. Which will have a higher Rf value in a non polar solvent and why?arrow_forwardLet's say that you found the perfect eluent for separating benzoic acid and naphthalene on a silica gel TLC plate. Which compound would have the higher Rf value after you eluted the plate? (Hint: look up the structures for both of these compounds). O A. It cannot be determined with the given information. O B. Benzoic Acid O C. Neither. They would have the same Rf value. O D. Naphthalenearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY