
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
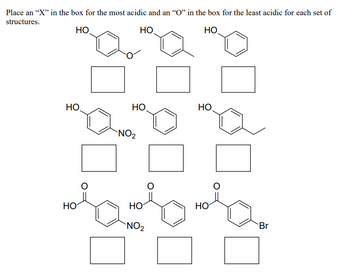
Transcribed Image Text:Place an "X" in the box for the most acidic and an "O" in the box for the least acidic for each set of
structures.
HO
НО
НО
НО.
НО
0
НО
NO₂
НО
NO₂
0
НО
НО
0
Br
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Rank the acidity of the molecules A through E, shown. Select the correct response from each of the pull-down menus. A HH N H3C H B The most acidic molecule is [Select] The second most acidic molecule is [Select] The third most acidic molecule is [Select] H3C The fourth most acidic molecule is [Select] The least acidic molecule is [Select] C H + H3C H D • H₂C E Harrow_forward3. Which of the following species is the strongest base? Multiple Choice HO H2N CH3CO0 CIarrow_forwardrank the following carboxylic acids baed in their pka please ( most acidi to least acidic)arrow_forward
- Which is the least stable base? (F-, Cl-, Br-, and I-)arrow_forwardWhy is the chlorine in the ortho position less acidic than the one in the para position? Please explain in detail.arrow_forwardExplain which compound is more acidic than the other. I II CF3 O OH OH I is more acidic because of resonance. II is more acidic because of induction. II is more acidic because of resonance. I is more acidic because of induction.arrow_forward
- Determine which of the following carboxylic acids will be the most acidic by looking at their structures and whyarrow_forwardSelect the strongest base. NaOH CH3CH₂CH₂CH₂Li O (CH3)2NK O Na₂CO3arrow_forwardAcidity: Rank ammonium, piperidinium, piperidine, and 1,3-dioxazacyclohexane in order of acidity Part A Rank the following compounds from most acidic to least acidic. Rank the compounds from most acidic to least acidic. To rank items as equivalent, overlap them. most acidic Submit ΝΗ Previous Answers Request Answer NH +H₂O NH₂ Review Reset Help least acidicarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY