
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please explain step by step and what formula is being used.
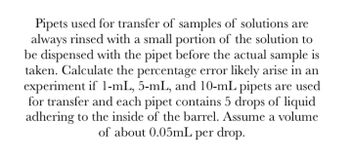
Transcribed Image Text:Pipets used for transfer of samples of solutions are
always rinsed with a small portion of the solution to
be dispensed with the pipet before the actual sample is
taken. Calculate the percentage error likely arise in an
experiment if 1-mL, 5-mL, and 10-mL pipets are used
for transfer and each pipet contains 5 drops of liquid
adhering to the inside of the barrel. Assume a volume
of about 0.05mL per drop.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please answer question 20 Part Aarrow_forwardMacmillan Learning Refer to the structures of molecules A-E. OH B molecule D. molecule C molecule E molecule A molecule B OH C Identify the molecules that will react with iron(III) chloride. CH3 OH D OH OH E CH3arrow_forwardThis is the chemical formula for hexanal: CH3CH24CHO. Calculate the mass percent of hydrogen in hexanal. Round your answer to the nearest percentage.arrow_forward
- Chemical formulas can be written as empirical formulas, molecular formulas, or structural formulas. Explain why these different forms of molecular expression are necessary.arrow_forward1. A compound (molar mass= 324.41 g/mol) is used as a cardiac depressant. It has this percent composition by mass. 74.04% C; 7.46% H, 8.64% N; and 9.86% O. Use these data to determine the empirical formula and molecular formula of that compound. 2. Give the family name (i.e noble gas, halogen, alkali, or alkaline earth metal) for the following elements: I) Calcium II) Bromine III) Potassium IV) Barium V) Neonarrow_forwardHow many H atoms are in one formula unit of (NH₄)₂SO₄?arrow_forward
- Calculate the molecular formula for the compound found to have the following elemental composition by mass: 40.0% C, 6.71% H, 53.3% O. The molar mass of the compound is 90.08 g/mol. I'm confused what to do next after acquiring the empirical formula mass.arrow_forwardWrite the correct chemical formula.arrow_forwardConsider the mixture of propane, C3H8C3H8, and O2 Write a balanced equation for the combustion reaction that occurs between propane and oxygen.arrow_forward
- write formula out. balancing not needed 1. tin (II) dichromate + lead (IV) sulfate -->arrow_forwardThe chemical structure of lactic acid (C,H,03) is shown below. Highlight each atom that is in a hydroxy group. H :0: ? Н —О C C -0. - - .. Н —с — Н H :0 :arrow_forward47.00 g sample of MgCO3.XH2O is heated to move away the water. After that, the total mass was reduced to 22.74 g. Determine the formula of the hydrate. Show your calculations.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY