
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
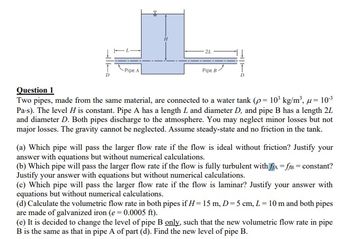
Transcribed Image Text:-Pipe A
2L
Pipe B
Question 1
Two pipes, made from the same material, are connected to a water tank (p= 10³ kg/m³, u = 10-³
Pa-s). The level H is constant. Pipe A has a length L and diameter D, and pipe B has a length 2L
and diameter D. Both pipes discharge to the atmosphere. You may neglect minor losses but not
major losses. The gravity cannot be neglected. Assume steady-state and no friction in the tank.
(a) Which pipe will pass the larger flow rate if the flow is ideal without friction? Justify your
answer with equations but without numerical calculations.
(b) Which pipe will pass the larger flow rate if the flow is fully turbulent with ffA=fB = constant?
Justify your answer with equations but without numerical calculations.
(c) Which pipe will pass the larger flow rate if the flow is laminar? Justify your answer with
equations but without numerical calculations.
(d) Calculate the volumetric flow rate in both pipes if H= 15 m, D=5 cm, L = 10 m and both pipes
are made of galvanized iron (e=0.0005 ft).
(e) It is decided to change the level of pipe B only, such that the new volumetric flow rate in pipe
B is the same as that in pipe A of part (d). Find the new level of pipe B.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps with 26 images

Knowledge Booster
Similar questions
- In some of Joule’s experiments (Figure 1), work was done on water held in an adiabatic calorimeter. The work was done by a rotating paddle, driven by falling weights. Assume the volume of the water remains constant during these experiments.a. In one experiment a 25-kg mass was allowed to fall 20 times through a height of 2 m; what was the maximum amount of work done? b. If a 25-kg mass were fired into the calorimeter and brought to a standstill, what should its initial velocity be to accomplish the same effect as in (a)? c. If the calorimeter held 1.2 kg of water and if process (a) caused the water temperature to rise from 288 to 290 K, what is the numerical value for the factor that connects temperature rise to work under these conditions?arrow_forward5arrow_forwardQ.3 A converging-diverging nozzle is supplied by a huge air reservoir. The nozzle has a throat area of 10 cm? and an exit area of 20 cm?. The reservoir conditions are P. = 300 kPa and To = 30 °C. Determine the range of the back pressure over which (a) shocks appear in the nozzle, (b) oblique shocks form at the exit, and (c) expansion waves form. (d) Calculate the mass flow rate through the nozzle for the case in which a normal shock appears at the exit plane.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The