
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
15 .
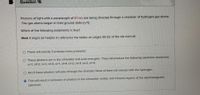
Transcribed Image Text:Question 16
Photons of llght with a wavelength of 97 nm are belng directed through a chamber of hydrogen gas atoms.
The gas atoms began in their ground state (n-1).
Which of the following statements is true?
Hint: It might be helpful to reference the tables on pages 90-92 of the lab manual.
O There will exactly 3 emission lines produced.
O These photons are in the ultraviolet and quite energetic. They willl produce the following electronic transitions:
ni=1, nf=2; ni=1, nf=3; ni=1, nf=4; ni=2, nf=3; ni=2, nf=4.
All of these photons will pass through the chamber. None of them will interact with the hydrogen.
This will result in emission of photons in the ultraviolet, visible, and infrared regions of the electromagnetic
spectrum.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the molar concentration (in citric acid) of a solution where 0.365 moles of citric acid are dissolved in 672 mL of water? (3 sf)arrow_forward9.71 Determine the number of moles of each of the following salts that would equal 1 eq of salt: Answer to 2 decimal places. For example 0.25 KNO3 moles Li2CO3 moles SrCl2 molesarrow_forwardIdentify the major organic productarrow_forward
- Which of the following species is the conjugate of ibuprofen? B C எள்ள்ள் Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a A b B c c d D A OH OH2 Darrow_forward10:24 1 Done Introductory+Chemistry ill a 10. What mass of Li₂O is needed to react with 1,060 g of CO₂? Li₂O(aq) + CO₂(g) → Li₂CO3(aq),arrow_forwardWhich of the following phosphate salts are insoluble in water? A. (NH4)3PO4 B. Li3Po4 C.Na3PO4 D.K3PO4 E.Ca3(PO4)2arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY