
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
What's the difference between LDA, NaH, -OH, and a weak base is this case?
Transcribed Image Text:Ph
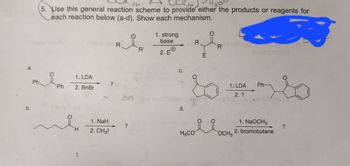
5. Use this general reaction scheme to provide either the products or reagents for
each reaction below (a-d). Show each mechanism.
Ph
1. LDA
2. BnBr
H
1. NaH
2. CH31
R.
?
'R'
1. strong
base
2. EⓇ
C.
d.
R
E
D
H3CO
O
'R'
요요
1: LDA
2. ?
OCH 3
mainersom
Ph
1. NaOCH3
2. bromobutane
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2:10 2- 3C₂²- + 1 2 3 4 N Complete the balanced chemical reaction for the following weak base with a strong acid. In this case, write the resulting acid and base as its own species in the reaction. NH3(aq) + HCl(aq) (s) Question 1 of 10 O Reset LO 5 OH H₂O 6 CI 7 6 个 x H₂O Tap here or pull up for additional resources Submit 8 9 11 (aq) O H H3O+arrow_forwardSodium hydrogen carbonate NaHCO3, also known as sodium bicarbonate or "baking soda", can be used to relieve acid indigestion. Acid indigestion is the burning sensation you get in your stomach when it contains too much hydrochloric acid HCl, which the stomach secretes to help digest food. Drinking a glass of water containing dissolved NaHCO3 neutralizes excess HCl through this reaction: HCl(aq)+NaHCO3(aq)→NaCl(aq)+H2O(l)+CO2(g) The CO2 gas produced is what makes you burp after drinking the solution. Suppose the fluid in the stomach of a woman suffering from indigestion can be considered to be 200.mL of a 0.089M HCl solution. What mass of NaHCO3 would she need to ingest to neutralize this much HCl? Be sure your answer has the correct number of significant digits.arrow_forwardI have 3.025 lb H2SO4 and 200 gal of water. How many molecules of sulfuric acid do I have? What is the pH? All hydrogen atoms convert to hydronium ions. 1 oz= 28.35 g 1 gall=3.785 Larrow_forward
- Calculate the pH and the pOH of these solutions: (a) 0.0017 M NaOH pH = pOH = (b) 0.158 M HCl pH = pOH = (c) 0.0277 M HC6H5O (Ka = 1.3 x 10-10) pH = pOH =arrow_forwardThe formula for the conjugate acid of HPO42- is? A student weighs out a 2.33 g sample of sodium fluoride, transfers it to a 300 mL volumetric flask, adds enough water to dissolve it and then adds water to the 300 mL tic mark. What is the molarity of NaF in the resulting solution? A student wants to prepare a solution of iron(III) fluoride with a known molarity.How many grams of FeF3 must be weighed out to prepare 250. mL of a 0.245 M aqueous solution of the salt? An aqueous solution has a hydroxide ion concentration of 1.0 x 10^-10 M. What is the hydronium ion concentration in this solution? Concentration = M Is this solution acidic, basic or neutral? An aqueous solution has a hydrogen ion concentration of 1.0 x 10^-6 M. What is the hydroxide ion concentration in this solution? Concentration = M Is this solution acidic, basic or neutral?arrow_forwardWrite the chemical formual of acid: Cl-(aq0+HS04-(aq) HCI(AQ+SO42-(aq)arrow_forward
- Calculate the concentration of OH ions in a 1.00 × 103 M HCl solution. Be sure your answer has the correct number of significant digits. Note: Reference the Fundamental constants table for additional information. [он ]= мarrow_forwardBe sure to answer all parts. Enter your answers in scientific notation. Calculate the hydronium ion concentrations of the following solutions at 25°C, given the pH. (a) pH = 9.20 %3D [H,0*]=Ox 10. (b) рH %3D 3.82 [H,0*] = x 10 Marrow_forwardWhat is the pH of an aqueous solution with a hydrogen ion concentration of [H] = 2.1 x 10-5 M? pH =arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY