
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:% Transmittance
50
(a) Compound A
100
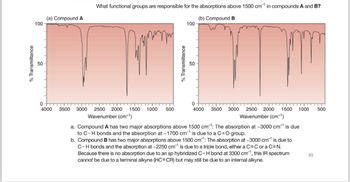
What functional groups are responsible for the absorptions above 1500 cm¹ in compounds A and B?
при
100
(b) Compound B
% Transmittance
50
0
0
4000 3500 3000 2500 2000 1500 1000 500
4000 3500
Wavenumber (cm-1)
3000 2500 2000 1500 1000
Wavenumber (cm¹)
500
a. Compound A has two major absorptions above 1500 cm-1: The absorption at ~3000 cm¹ is due
to C-H bonds and the absorption at -1700 cm¹ is due to a C=O group.
b. Compound B has two major absorptions above 1500 cm: The absorption at -3000 cm¹ is due to
C-H bonds and the absorption at -2250 cm is due to a triple bond, either a C=C or a C=N.
Because there is no absorption due to an sp hybridized C-H bond at 3300 cm, this IR spectrum
cannot be due to a terminal alkyne (HC=CR) but may still be due to an internal alkyne.
49
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Need all IR and NMR assignments and the structure of the compoundarrow_forwardFind the structural shape of the compound according to the infrared spectrum?arrow_forwardFigures below give a molecular formula, an infrared spectrum,and a 1H NMR spectrum for an unknown compound.What is the structure of the compound? Explain briefly.arrow_forward
- Could you please interpret this? I know it is a very weak alcohol, but I’m unsure beyond that.arrow_forwardName the major IR absorptions and write the name of the unknown compound and its structure.arrow_forwardInterpet and analyze this Infrared (IR) spectra to show the signs from the peaks and what they tell about the unknown compound.arrow_forward
- Which of the shown compounds corresponds to the shown IR spectrum ? Micrometers 2.5 100 8 10 12 13 14 15 20 90 80 70 50 40 30 20 10 4000 3600 3200 2800 2400 2000 1800 1600 1400 1200 1000 800 600 400 Wavenumber (cm-!) NH2 Transmittance (%)arrow_forwardDetermine the compound (name or structure) from the data. Explain features from each data. Molecular formula: C6H5Br Use molecular formula to determine IHD IR: Identify the presence/absence of five key functional groups NMR: Analyze this last. Consider multiplicity and peak area to confirm compound. The 5 H peak area covers both peaks (at 7.1 and 7.5 ppm Structure?arrow_forwardDraw the structure of the acid chloride with a molecular formula of C8H7ClO and the following spectroscopic data:arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY