
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
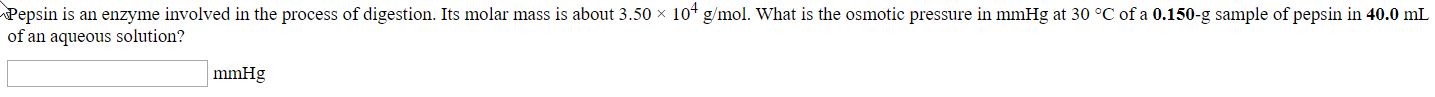
Transcribed Image Text:Pepsin is an enzyme involved in the process of digestion. Its molar mass is about 3.50 x 10 g/mol. What is the osmotic pressure in mmHg at 30 °C of a 0.150-g sample of pepsin in 40.0 mL
of an aqueous solution?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 6 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- At what temperature would a 0.2910 M solution of magnesium sulfate (MgSO4) in water exhibit an osmotic pressure of 15.10 atm? Assume magnesium sulfate completely dissociates. R = 0.08206 L atm/K.molarrow_forwardWhen 0.713 grams of a protein were dissolved in 72.5 mL of solution at 28.5 degrees C, the osmotic pressure was found to be 35.6 torr. Calculate the molar mass of the protein.arrow_forwardV A student dissolves 10. g of aniline (C6H5NH₂) in 425. mL of a solvent with a density of 0.98 g/mL. The student notices that the volume of the solvent does not change when the aniline dissolves in it. Calculate the molarity and molality of the student's solution. Round both of your answers to 2 significant digits. esc = 10 molality = 0 molarity = Explanation Check 010 # 3 X 24 $ 4 0×0 3 % 5 MacBook Pro 6 & 7 2022 McGraw Hill LLC. All Rights Reserved. ● * 8 Terms of Use | Privacy Center | Accessibility 0 A + 11 olo Ar 18 ?arrow_forward
- What is the osmotic pressure, in atm, of a 0.183 M solution of MgCl₂ at 37.0 °C? (assume complete dissociation).arrow_forwardWhat is the osmotic pressure, in torr, of a solution formed by dissolving 0.2917 g of ammonium perchlorate in enough water to make 103.6 mL of solution at 37.83 °C? Assume ideal behavior.arrow_forwardWhat is the osmotic pressure of a 4.5 FL OZ solution containing 30.6 g of fructose (C6H12O6) at 40 °F. Osmotic pressure is 29.2 atm. Explain why.arrow_forward
- Please provide detailed solution and give the explanation of the concept Calculate the osmotic pressure of a solution containing 19.25 mg of hemoglobin in 14.7 mL of solution at 35 °C. The molar mass of hemoglobin is 6.5 x 10^4 g/mol. Express your answer in atmospheres.arrow_forwardWhat is the molar mass of a protein if 5.87 mg per 10 mL gives an osmotic pressure of 2.45 torr at 25 °C?arrow_forwardThe molar mass of an enzyme was measured by dissolving the 3.221 grams of the enzyme in 1.00 liter of water. The osmotic pressure of the solution was 5.746 cm of solution at 20°C (assume the density of the solution is 1.00 g/mL and the density of mercury is 13.56 g/mL). What is the molar mass of the enzyme?arrow_forward
- An aqueous solution containing 10.1 g of starch per liter has an osmotic pressure of 3.60 mm Hg at 25 °C. a What is the average molar mass of starch? (Because not all starch molecules are identical, the result will be an average.) g/mol Molar mass=arrow_forward10.0 g ZnCl2 is dissolved in 100.0 g of water at 23.6 °C. The solution reaches a temperature of 36.5 °C. What is the molar heat of dissolution for ZnCl2? Assume the specific heat capacity of the solution is the same as pure water, 4.184 J/g·°C.arrow_forwardWhat is the solubility of potassium chloride at 20 degrees Celsius?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY