
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
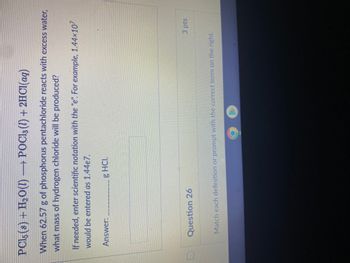
Transcribed Image Text:PCl5 (s) + H₂O(l) →→→ POCl3 (1) + 2HCl(aq)
When 62.57 g of phosphorus pentachloride reacts with excess water,
what mass of hydrogen chloride will be produced?
If needed, enter scientific notation with the "e". For example, 1.44×10²
would be entered as 1.44e7.
Answer:
g HCI.
Question 26
3 pts
Match each definition or prompt with the correct term on the right.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- You are performing an experiment in lab that involves the titration of a H2SO4 solution. You titrate the acidic solution with 0.5166 M NaOH and the equivalence point is reached by the addition of 21.76 mL of NaOH solution. Using the balanced equation below, calculate the moles of H2SO4 in the flask. Do NOT include units or write the answer in scientific notation. 2NaOH(aq) + H2SO4(aq) → 2H2O(l) + Na2SO4(aq)arrow_forwardA CHEM 1151K student notices that it takes 20.0 mL of a 1.5 M solution of KOH to completely neutralize 25.0 mL of carbonic acid (H2CO3). What is the molarity of the acid?H2CO3 (aq) + 2KOH (aq) -----> Na2CO3 (aq) + 2H2O (l) 0.80 M H2CO3 0.40 M H2CO3 1.8 M H2CO 0.60 M H2CO3arrow_forwardWrite net ionic equation (NH4)2SO4(aq) + PbCl2(aq) --------> PbSO4(s) + 2NH4Cl(aq)arrow_forward
- According to the following reaction, how many milliliters, mL of 0.152 M HCI is needed to neutralize 2.47 grams of Ca(OH)2? Just give the numerical value. The assumption is the answer is in milliliters, mL. Reaction: CaCl₂(aq) 2 HCl(aq) + Ca(OH)₂(aq) --- 2 H₂O(l) +arrow_forwardWhat mass (in kg) of H(OH) can be produced if 56mL of 0.982M H2C2O4•H2O solution reacts completely with excess NaOH? __ H2C2O4•H2O + __NaOH = __ Na2C2O4•H2O + __ H(OH)arrow_forward27.arrow_forward
- 0.1061 g of dissolved oxalic acid dihydrate (molecular weight = 126.064 g/mole) is reacted with 20.0 mL of a sodium hydroxide solution. Calculate the molarity of the sodium hydroxide solution. The balanced equation can omit the dihydrate from oxalic acid. Group of answer choices 0.0421 M 1.34 M 0.334 M 0.0842 M 0.669 Marrow_forwardIf 2.00 grams of an unknown DIPROTIC acid (H2A) is titrated with 34.0 mL of 1.35 M NaOH, what is the molar mass (g/mol) of the diprotic acid? H2A+2NaOH⟶2H2O+Na2AH2A+2NaOH⟶2H2O+Na2A (Hint: See equation below in which mm is molar mass (units: g/mol), m is mass (unit: g), and mol isnumber of moles (unit: mol)). mmacid=macidmolacidmmacid=macidmolacid Molar Mass = ___________ g/mol (input the numeric value only!) Remember: use correct significant figure rules. Significant figures always matter in Chemistry!arrow_forwardQuestion 34 of 42 If 32.0 g of NaOH is added to 0.550 L of 1.00 M Ni(NO,),, how many grams of Ni(OH), will be formed in the following precipitation reaction? 2 NaOH(aq) + Ni(NO,),(aq) → Ni(OH), (s) + 2 NaNO, (aq) 1 4 7 8. +/- x 100 MacBook 21arrow_forward
- Please helparrow_forwardConsider the unbalanced equation for the neutralization of acetic acid: HC2H3O2 (aq) + Ba(OH)2 (aq) ⟶ H2O (l) + Ba(C2H3O2)2 (aq) Balance the equation and determine how many moles of Ba(OH)2 are required to completely neutralize 0.465 mole of HC2H3O2. Mol Ba(OH)2arrow_forwardsolution after precipitation is complete. 68. The drawings below represent aqueous solutions. Solution A is 2.00 L of a 2.00-M aqueous solution of copper(II) nitrate. Solution B is 2.00 L of a 3.00-M aqueous solution of potas- sium hydroxide. (0) suiro Cu²+ ebro NO3 БО K+ OH™ A B a. Draw a picture of the solution made by mixing solutions A and B together after the precipitation reaction takes place. Make sure this picture shows the correct relative volume compared to solutions A and B, and the correct relative number of ions, along with the correct relative amount of solid formed. b. Determine the concentrations (in M) of all ions left in solution (from part a) and the mass of solid formed.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY