
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Which ions remain in solution after PbI2 precipitation is complete?
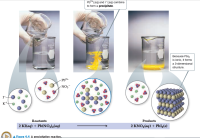
Transcribed Image Text:Pb2*(aq) and I (aq) combine
to form a precipitate.
150
150l
150
Because Pbl2
is ionic, it forms
a 3-dimensional
structure.
Pb2+
NO3
I-
K+
Reactants
Products
2 KI(aq) + Pb(NO3)½(aq)
2 KNO3(aq) + PbI_(s)
Figure 4.4 A precipitation reaction.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How many equivalents are in 15.0 mL of 0.12 M Ba(OH)2 solution? What volume of 0.085 M HNO3 is required to reach the end point when titrating 15.0 mL of this solution?arrow_forwardWhat precipitate is produced from this reaction? MgCl2(aq) + NaOH(aq) → Question 7 options: MgOH NaCl nonearrow_forward10.5 mL of 0.139 M Mg(NO3)2 is mixed with 11.9 mL of 0.625 M NaOH. Calculate the final concentration (in M) of hydroxide ion ([OH-]) after any reaction that occurs goes to completionarrow_forward
- An unknown was treated with six drops of HCI. A precipitate was observed. Which of the following ion(s) could be present? Pb²+ Ag+ Fe2+ 2+ H82arrow_forwardIf 550 mL of some Pb(NO3)2 solution is mixed with 100 mL of 7.50 x 10−2 M NaCl solution, what is the maximum concentration of the Pb(NO3)2 solution added if no solid PbCl2 forms? (Assume Ksp = 2.00 x 10−5 M at this temperature.) Enter the concentration in M.arrow_forwardA 5.50 mL sample of an H3PO4H3PO4 solution of unknown concentration is titrated with a 1.050×10−2 MNaOH solution. A volume of 7.32 mL of the NaOH solution was required to reach the equivalence point. What is the concentration of the unknown H3PO4H3PO4 solution? Express your answer with the appropriate units. value/unitsarrow_forward
- 2. Using the procedure described in this module, a student determined the percent KHP in an impure sample of KHP. A 3.150-g sample of impure KHP required 41.50 mL of 0.1352M NaOH solution for titration. (a) Calculate the number of moles of NaOH required for the titration. (b) Calculate the number of moles of KHP present in the impure sample of KHP. (c) Calculate the number of grams of KHP present in the impure sample. (d) Calculate the percent of KHP in the impure sample, using Equation 8. Equation 8: percent KHP in the impure sample, % = ( mass of KHP in the sample,g/ mass of sample analyzed, g) (100%)arrow_forward40 mL of LiOH are titrated to the equivalence point with 65 mL of 1.2 x 10-2 M HNO3. Find the concentration (molarity) of the LiOH solution.arrow_forwardIf a 0.11 g sample of a weak acid requires 25.30 ml of 0.1036 M NaOh to reach an equivalence point, what is the molar mass of the acid?arrow_forward
- helparrow_forwardQuestion 4: After the student made the working NaOH solution above, he conducted a titration of an HCl solution of unknown concentration. If the student used a 20.5 ml aliquot of the HCl solution and used 22.7 ml of the NaOH solution from #3 above to reach the endpoint, what is the concentration of the HCl solution?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY