
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
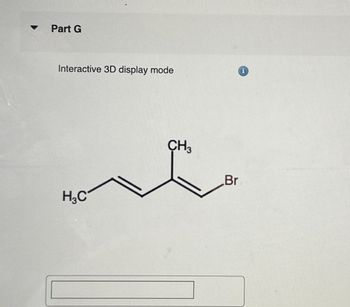
Transcribed Image Text:Part G
Interactive 3D display mode
H3C
CH3
Br
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- 2. 100 12 133 134 1 0 С6H8O 4000 9 HSP-45-691 8 3000 1 : 7 1 T 6 2000 5 ppm NAVENUMBERI : 4 T 3 1500 4:2 T 2 T T 1 wwwm 1000 0 500 DEPT-90 DEPT-135 - 200 180 160 140 120 COS-11-743 لد 100 ppm 80 60 40 20 m 0arrow_forwardMuch of Earth's history can be unraveled studying sulfur. Sulfur can be used as "geothermometer". The ratios of stable sulfur isotopes change with the temperature of Earth's processes. For example, igneous systems deep inside the Earth occur at very high temperatures, hydrothermal systems occur at intermediate conditions, and sedimentary rock weathering occurs at low temperatures. The composition of stable sulfur isotopes varies across the 1000 degree temperature range. 10. How many peaks would be seen in the mass spectra for such a study? Make a basic sketch (bar chart) a.m.u. vs % natural abundance and label each peak's a.m.u. and % natural abundance.arrow_forwardPredict all the chemical compounds in the table below from the given chemical formula and other physical properties Chemical Name Chemical Formula M (g/mol) Boiling Point Melting Point Refractive Index (power of n to D) (nD) C6H14 86.18 69 1.375 SiO2 60.1 1600-1725 C13H10 166.22 295 C13H8O 180.19 342 1.6309 C3H6O 58.08 57 1.35900arrow_forward
- 1) SOCI₂, Et3N OH 2) + AICI 3arrow_forwardCH3 Brz _Z1g + उarrow_forwardPlease choose an appropriate technique(s) to separate following compounds (it can be one, more than one, on none): Compound Melting Point Density Boiling Point Molar mass Phenol 40.5°C 1.07 g/cm 181.7°C 94.113 g/mol Toluene -95 °C 0.87 g/mL 111 °C 92.141 g/mol Simple Filtration Column Chromatography Distilation Crystallization/Re-Crystallization Separatory funnel None www. MacBook Airarrow_forward
- 11. A material safety data sheet (MSDS) for a chemical is shown below. MSDS H3PO4(aq) Section 9: Physical and Chemical Properties Physical state and appearance: Viscous liquid Odor: Odorless Color: Clear, colorless Boiling point: 158°C Melting point: 21°C Specific gravity: 1.685 at 25°C Which of these is the appropriate name for H3PO4 (aq)? A. Trihydrogen phosphite B. Phosphoric acid C. Phosphorous hydroxide D. Phosphorous acidarrow_forward2. Explain how suction filtration is carried out in the laboratory. Give the important points that must be observed in doing the process.arrow_forwardBr [A] fins [B] Br [C] 17. Discuss the relationship between Compounds A, B and C. Then draw a diagram to show what you would expect to see on a TLC plate if you compared the three compounds on the same plate. 120m 9marrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY