
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%

Transcribed Image Text:lipoul
biq priwoll
Part D: Mass and Volume
1. Number a set of 1.5 mL conical microtubes 1-9.
2. Determine the mass of each tube.
3. Use the 2- 20 µL pipette to deliver 3 different volumes of DI water within that range to
tubes 1, 2, and 3.
4. Use the 20-200 µL pipette to deliver 3 different volumes of DI water within that range to
tubes 4, 5, and 6.
5. Use the 100 - 1000 µL pipette to deliver 3 different volumes of DI water within that range
to tubes 7, 8, and 9.
6. Determine the mass of each tube with the water added.
7. Graph the mass of the water vs. the volume of the water (pipette set volume). Determine
the best fit line for the data and the associated linear equation.
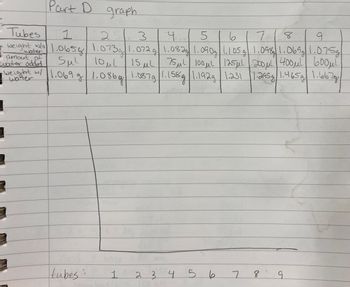
Transcribed Image Text:Part D
graph
Tubes
1
125 3 4 5
6
718
9
w/o
amout of
water added
weight for 1.065g 1.073g|1.072 g 1.08.2g 1.090g|1.105 g 1.098 1.069g| 1.075g
Sμl 10μl 15 μl 75 μl 100μl 125μl 200μl 400μl b00μl
weight w/ 1.069 g 1.086g 1.087g|1.158g|1.1929 1.231 |1.295g|1.465g|| 1.6678
|
tubes:
19
1
2 3 4 5 6
7 8 9
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Similar questions
- Nonearrow_forwardIf 2.4 vial Conatins 100 units of Botox and 0.2 ml is injected into 3 sites during surgery how many units will remain in the vialarrow_forwardYou are asked by the pharmacist to add 45 mEq of Ca Gluconate in an IV bag of D5W 1000mL. You have a concentrated vial of Ca Gluconate 4.4 mEq/mL, 50 mL. How many mL of this concentrated vial needs to be added to the IV bag?arrow_forward
- Units of volume 26 0.15 litres (L) to millilitres (mL)arrow_forwardpH 3.5 4.5 5.5 6.5 7.5 8.5 Absorbance 0.098 0.027 0.068 0.028 0.032 0.054 Concentration in diluted supernatant (mg/ml) 0.196 0.054 0.136 0.0056 0.0064 0.0108 Concentration in undiluted supernatant (mg/ml) 0.98 0.27 0.68 0.28 0.32 0.54 % solubility Formula for % solubility of proteins: % solubility = [(C x V x F) / (1000 x 35%)] x 100% C = concentration (mg/ml); F = dilution factor; V = volume of solution (ml) Calculate the % solubility of protein in the soy flour (Assume the soy flour contains 35% protein (w/w))arrow_forwardThe drug premarin is found In scered tablets of The 1:25mg. drug orIered is Premañin 312.5mcg. Huw much wareld you give?arrow_forward
- Four lab groups completed the experiment listed in the lab diluting lul of FCF dye into 1ml of water. The A625 values are listed below. For brilliant blue FCF, ɛ =97000 M-1cm–1 MW = 792.8 g/mol %3D Group # A625 % solution (in original stock sample) Percent ror (True value of Stock=0.2%) 1 0.244 0.199 0.5 0.209 0.170 15 3 0.306 0.250 25 4 0.187 0.153 0.235 a. Which group was more accurate? b. What are possible reasons group 3 had a much higher Absorbance than the others? c. What are possible reasons group 4 had a much lower Absorbance than the others?arrow_forward2:20 PM Wed Mar 23 7 82% T (24) Doctor. G ask you to prepare lidocaine 2mg per minute IV for a patient. You have a solution of 1.2g Lidocaine in 950mL D5W. How many milliliter are you giving per hour?arrow_forwardAn order arrives in the pharmacy for K PO4 30 mEq to be added to each liter of normal saline. You have in stock a vial with 4.4 mEq/mL. How many mL will you place in each IV bag?arrow_forward
- 12. 108 cm = inarrow_forwardTime (min) 0 15 30 45 60 75 Solution A Weight (8) 4.205 4.032 3.787 3.648 3.550 3.500 Change (%) Solution B Weight (8) 3.611 -4.11% 3.606 -6.07% 3.622 -3.67% 3.662 -2.69% 3.706 1.41% 3.732 Change (%) Solution с Weight (8) 4.257 -0.138% 4.213 0.444% 4.219 4.209 Change (%) 4.210 -1.03% Solution D Weight (8) 4.728 4.513 0.142% 4.350 1.10% 0.237% 4.254 1.20% 0.024% 4.068 0.675% 4.209 -0.024% 4.140 Change (%) -4.55% -3.61% -2.21% -4.37% 1.77% Graph your results below, plotting percent weight change versus time for all 4 unknown solutions on one set of axes, labeled clearly with units. Include a title for your graph.arrow_forwardPgDn Ctrl Ins Del NW113AACE04–21 6. A student used Dumas procedure to calculate the Molar mass of a volatile liquid and collected following data. Calculate the molar mass of the volatile liquid using the data given Temperature of the water bath = 100.25 °C Mass of water remaining after condensation = 0.556 g Atmospheric pressure = 750 mmHg Volume of the Flask = 255 mLarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON